Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 22 February 2026, 12:19 AM
Module 5: Germination and dormancy
Introduction
Welcome to module 5. Earlier in this course, you learned about germination testing and how it helps you to monitor viability. This final module supports you in the practicalities of germination testing, as well as giving a fascinating glimpse of new technologies that may help us predict the ability of seeds to germinate.
You will learn how to maximize the chances your seeds will germinate, how seeds that do not germinate may provide useful information about the quality of your seeds, and the effectiveness of your testing. Dormancy, a failure to germinate even when environmental conditions are ideal, was first introduced in module 1. By the end of this module, you will understand the different mechanisms of dormancy that exist in the natural world, and what you can do to break these types of dormancy.
When your viability data tells you it is time to regenerate, it is crucial to ensure that the next generation of seeds is as close as possible to the genotype of the seeds in the original accession. This module gives you helpful tips on how to ensure that the samples you keep from the new generation of seeds are as close as possible to the original samples.
By the end of this module, you should be able to:
- List the factors required for germination.
- Give examples of how substrate, water, temperature, light and gaseous environment can be optimized in germination testing.
- Discuss the importance of genetic integrity to your genebank’s mission, and what you can do to conserve it.
- Estimate rates of germination over time.
- Describe the different types of dormancy that occur in wild species.
- Explain, in a general way, how to break common types of dormancy.
- Evaluate the procedures in your own workflow.
Overview of genebank processes
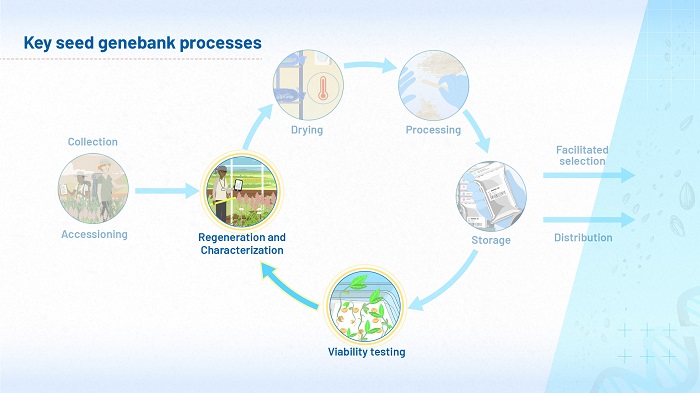
This module focuses on the biology of germination and dormancy. It will help you to successfully carry out viability testing and regeneration, the two processes highlighted in Figure 1.
A crucial objective in the management of seeds is to ensure they are able to germinate. Genebanks invest a lot of resources in viability testing: it begins soon after the seeds arrive, before they are packed and placed in the genebank, and continues with regular germination tests while the seeds are in storage. To what extent can the results of all this testing be relied upon?
If a germination test indicates that viability is low, the seeds must be regenerated, yet the process of regeneration can bring its own risks. Depending on the species, it is possible that the new generation of seeds could be different from the original accession, perhaps because of cross-pollination, genetic drift, pollen contamination, seed contamination or even mislabeling. Here, we consider ways to carry out regeneration that will help to ensure the characteristics of the next generation of seeds are as close as possible to those of the original sample.
Dormancy is one reason why seeds may appear to fail to germinate, when in fact they are perfectly capable of doing so. By mistaking dormancy for low viability, you could initiate unnecessary cycles of regeneration. In some species, dormancy may be broken by the cold, dry conditions of storage. In others, dormancy may be broken by heat or chemicals. There are many types of dormancy, but they can be overcome. By the end of this module, you will appreciate the main mechanisms of dormancy, and some common laboratory techniques for tackling it.
Some definitions
A dead seed is a previously viable seed that has died.
An immature seed is one that has been collected in the early days after pollination, and is not naturally fertile (although it may under specific circumstances be cultured artificially).
A dormant seed is in a state of minimal metabolic activity. It is not able to germinate, even when environmental conditions are favourable, because something else needs to happen to break its dormancy.
A quiescent seed is a non-dormant seed that cannot germinate because environmental conditions are not favourable.
An empty seed is non-viable, because it has no ‘germ’ that is able to grow; it is not dead because it was never alive.
Regeneration is the renewal of germplasm accessions, for instance by sowing seeds then harvesting seeds from the resulting plants. The aim is to create a new generation of viable seeds, with the same characteristics as the original population.
Maintaining genetic integrity means ensuring that regenerated samples stored and distributed by a genebank are exactly the same as the original sample.
Genetic drift can occur when new generations of seeds deviate from the genotype that was originally collected, perhaps due to cross-fertilization, or contamination of seeds during threshing.
Conducting germination tests

There are different ways to test seed viability, but germination testing is the most accurate method. It determines what proportion of seeds in an accession will germinate under favourable conditions, and produce seedlings judged as normal according to specific criteria for each species given by the International Seed Testing Association (ISTA). As you learned in module 2, measuring the viability of seeds requires you to count germinated, un-germinated and abnormal seedlings at various points over a period of days or weeks.
Seeds of different species have different requirements. While some seeds can germinate under a wide range of conditions, in other species, germination can only be achieved under perfect conditions.
If a seed does not germinate during a germination test, there could be various reasons for this. The failure to germinate could be because of some mechanism within the seed, or environmental factors outside it. The seed could be immature, dead or empty. By dissecting a seed that failed to germinate, you can tell which of these apply. Alternatively, the failure to germinate may be because the seed is quiescent. If the seed is quiescent, it may still be perfectly capable of germinating in a more suitable environment.
In the next activity, you will start to unpack what we mean by ‘a suitable environment’. Have a go at the poll, which asks you to rate different aspects of a seed’s surroundings, in terms of how important you think they are for germination. In reality, of course, the factors that influence germination are highly species-specific, but for the purpose of this poll, we encourage you to think about generalities.
POLL: you decide
What do you think are the most important factors for germination?
Let’s see what you and your course colleagues think. Please express your opinion by following this link to the poll.
The results of this poll will be shared with your course colleagues and may be discussed at the next live event.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Germination requirements: substrate
Many seeds will germinate on paper or sand. Water is essential, whichever of these is used. As you learned in module 4, water is required for imbibition and many of the chemical reactions necessary for growth can only take place in solution. There should be enough water on the substrate to allow the seeds to imbibe prior to germination, usually by taking water in through the seed coat.
Once germination starts, the substrate must support the seeds and the emerging radical and cotyledons. It should be easy for you to separate out the germinated seeds when you need to inspect them. It is a good idea to seal the container, which could be a Petri dish, sandwich box or other container. If the container is not sealed well enough to maintain moisture levels for the duration of the germination test, it is important to add water to the substrate at regular intervals.
Some crops, such pearl millet, chicory and rye grass, are likely to germinate best when placed on top of a paper substrate, like the rye grass in Figure 2 (below):

Other seeds, such as oats, chickpea, black gram or rice, will germinate best if rolled up in moist paper, so the seeds are surrounded by substrate. Figure 3 shows some paper toweling rolls containing oat seeds, stacked vertically in a basket within a germinator. Opening up one of the rolled papers reveals the germinated seedlings as shown below:


Some crops such as onion or cabbage are commonly germinated on pleated paper. Folding the paper towel separates the seeds into rows; the pleated paper is then placed in a sealable box and kept moist while the seedlings germinate, like the Brassica seedlings in Figure 4 (below):

Germination requirements: gaseous environment
The vast majority of seeds need oxygen for germination. In most cases, if you reduce the amount of oxygen around a seed, or increase the amount of carbon dioxide, the rate of germination will drop. In module 3, you followed the development of a soybean seed, and saw what an energy-intensive process it is. The cell division required for the embryo and the cotyledons to form takes a huge amount of energy, making very high demands on the surrounding oxygen supplies. Like water, the oxygen required for respiration is usually taken in through the seed coat.
It might be tempting to think seeds also need carbon dioxide, since adult plants do, but in most cases, carbon dioxide is only useful to a seedling after photosynthesis has started, which happens after germination.
Case study: bucking the trend

Not every environment where plants germinate is rich in oxygen. In some places, such as the aftermath of flash floods during the monsoon, water stands stagnant and oxygen-starved for long periods. This presents a real challenge for seeds that can only germinate aerobically. Nevertheless, some submersed aquatic species have developed an alternative way to germinate, even in the absence of oxygen, called anaerobic germination. They do this using an alternative metabolic pathway.
These kinds of oxygen-depleted conditions are often found in rice production areas. Certain rice cultivars have the potential for anaerobic germination – a potential win for farmers’ ability to grow rice in places where flooding is increasing due to climate change. However, this ability to germinate under anaerobic conditions is the exception, not the rule.
Germination requirements: temperature
Seed growth only occurs within a certain range of temperatures. If the temperature is too high, the enzymes needed for germination are destroyed. If the temperature is too low, molecules inside the seed move too slowly for chemical reactions to get started. For any seed, there are three key temperatures, known as the cardinal temperatures, which describe the effect of temperature on germination:
- Tmin is the minimum temperature, below which none of the seeds from a sample will germinate.
- Topt is the optimal temperature, where there is high and fast germination.
- Tmax is the maximum temperature, beyond which none of the seeds from a sample will germinate.
Figure 5 (below) shows the results of an experiment where germination rates were compared at different temperatures. The successfully germinated seeds were counted and plotted early in the experiment (the red curve). Then, when germination appeared to have finished, successfully germinated seeds were counted and plotted again (the black curve).
Activity 1
Allow 5 minutes for this activity
Using Figure 5, estimate Tmin, Topt and Tmax for this particular species. Write your answers down in the boxes before checking our answers.
When you are ready, press 'reveal' to see our comments.
Discussion
You should have read off temperatures from the final germination (black) curve, although you may have found the first results (red) curve informative. Depending on what sort of screen you are viewing the graph on, your answers should be somewhere in the region of:
- Tmin = 7°C
- Topt = 25°C
- Tmax = 38°C
Different species of seed have different optimal temperatures. If you are dealing with a seed you have not encountered before, there are clues in the climate and conditions in the type of habitat the plant originates from. As a rule of thumb, for accessions of temperate origin, try conducting germination tests at constant temperatures between 15°C and 20°C. For accessions of tropical origin, consider constant temperatures between 20°C and 25°C.
Alternating temperatures
Some seeds are even more specific about temperature requirements. Figure 6 (below) shows how some species, in their natural environment, experience a diurnal rhythm, where temperatures change from day to night. Seeds near the surface of the soil are most likely to experience diurnal variations in temperature, whereas seeds buried deep in the soil experience a more constant temperature.
Small seeds with very small food reserves, which germinate on the surface of soil, and the seeds of plants from wetland habitats are among those most likely to benefit from alternating temperatures in germination tests. Seeds that tend to be buried deep underground in nature are more likely to germinate at fixed temperatures, because temperatures deep down in the soil do not alternate as much.
If you are working with species that are adapted to diurnal rhythms, and you mimic this pattern of alternating temperatures, germination tests may be more successful. For instance, watermelon seeds will germinate best when temperatures alternate between 20˚C for 16 hours per day (or 24 hour period) and 30°C for 8 hours.
Germination requirements: light

Most seeds germinate well in the dark. However, some small-seeded plants, such as lettuce, are adapted to growing on the surface of the soil: these do require light to germinate.
Seeds that require light may be sensitive to a minimum duration, intensity and especially quality of light. The wavelength can make a difference too – in some seeds red light promotes germination and blue or infra-red inhibits it. When working with light-sensitive species, it is usual to give 8 or 12 hours of light per day (24 hour period), with the light exposure corresponding with the warm phase if you are alternating the temperature as well.
The source of light can make a difference too: it is best to use low energy white fluorescent tubes or LEDs. Incandescent lamps can give out too much far-red light and heat. Of course, once the cotyledons have emerged and the seedling starts to photosynthesize, the presence of light takes on a whole new significance.
Germination requirements: time
Not all seeds in a germination test will germinate at the same time, and it is important to give seeds that do not germinate immediately the chance to germinate over a longer period.
In module 2, you explored seed survival curves for the viability of seeds in storage, and learned how these patterns can be modeled to make predictions. Just as patterns of seed viability change over time in a predictable way, it is also possible to describe patterns of seed germination using mathematical formulae. Figure 7 (below) draws on the work of Joosen et al., who modeled the germination of thale cress, Aridopsis thaliana, over time:
- Gmax is the final number of seeds that germinated at the end of germination testing.
- t50 is the time it takes for half of the seeds to germinate.
- U7525 is uniformity - the time to go from 25 to 75% germination.
Figure 7 mirrors what happens in the real world, for instance, t50 and U7525 can help you plan when to score germination. The area under the curve, AUC, provides useful information about both the speed and the final germination rate.
Pause for reflection
The beauty of mathematical modeling of germination is that it allows the possibility of predicting the number of seeds that can be expected to germinate at any particular point in time. In future, procedures involving visual imaging and machine learning could take the drudgery out of germination testing, by automating scoring. Although these processes are still experimental, in future they could allow high-throughput scoring of germination tests. Take five minutes to think about how this technology could affect genebanks.
Scoring germination
It is good practice to score germination tests and keep careful records of how many seeds have germinated normally, how many have germinated abnormally, and how many required some kind of intervention to encourage germination. The germination test will continue until all germination stops: the time this takes will depend on the species you are dealing with (and some wild species can take many months to germinate!).
Watch this video, a short clip from the longer video entitled ‘monitoring viability’ that you first watched in module 2. Now that you have considered germination in more depth, you should be able to approach the methodologies used by the two genebanks with a deeper understanding. As you watch, think about what decisions have been made by the two genebanks about how to carry out germination tests, and how these decisions relate to the underlying biology of the two crops.

Transcript: Video 1: Germination testing
Please write your comments on how this viability test is carried out. You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
In IRRI, they use a between papers (BP) approach. In IITA, the cowpeas are laid on top of papers (TP). In both genebanks, temperature and humidity levels are optimized for the crop: this is achieved in species-specific ways. In both genebanks, the scientists score germination by counting and discarding the germinated seeds. They keep careful records about numbers of seeds that germinate correctly, abnormally, or not at all. In IITA, scarification is used to encourage seeds that do not germinate, in order to maximise their chances of successful germination. Scarification is not necessary for cultivated rice, although it is used by IRRI for wild relatives of rice.
What is ‘normal’ growth?

In the video, the scientists examined the germinated seeds carefully. If they are to be useful to breeders and other users, the seedlings must be free from growth defects. Only seeds that have germinated correctly can be counted as being of good enough quality.
In the ISTA rules, the ‘germination percentage’ is calculated based on the number of sown seeds that have produced seedlings classified as ‘normal’ and that are able to go on to produce a fertile plant. Sometimes, seeds may germinate, but only in a form that is not compatible with life, for instance if only the cotyledons have emerged, not the radical. Various abnormalities may be seen, depending on the type of seed: deformed seedlings, fractures, discoloration, spindly stems, etc.
It is beyond the scope of this course to go into detail on what is classified as ‘normal’ germination for every type of seed – for this, the ISTA guidelines, listed in the ‘Useful publications’ section at the end of this module, are the best source. If you ask, you may find someone in your genebank who is able to share the information that ISTA provides on your specific crops.
The cut test
At the end of your germination test, if all goes well, the number of viable seeds will be above the Genebank Standards’ viability threshold (85%). But what if the results do not go the way you wished? Does that mean the seeds are no longer viable, and need regeneration? That’s possible, but there could be other reasons for apparently low germination rates, which require different courses of action. Ask yourself the following questions:
Questions about low germination rates:
- Was my method adequate?
- Did I give the seeds all the conditions they need to germinate?
- Are there empty seeds in the seed lot?
- Is the seed lot of low quality?
This is where a cut test provides useful information. Cut tests are examinations of seed anatomy: they involve dissecting longitudinally through any seed that has not germinated, and observing what state the cotyledons and embryo are in. By carrying out a cut test, you may gain valuable insights into what actions you need to take to improve seed quality. Record the number of seeds that are:
- Immature: small cotyledons or under-developed embryo (reduced chance of germinating)
- Ungerminated: firm, usually white tissues (usually viable but may have died in storage)
- Empty (never had a chance)
- Moldy: necrotic, often brown tissues (dead)
- Infested (dead)
Cut tests are an important decision tool for upgrading seed processing processes. For instance, if you find significant numbers of seeds were immature, you might be able to make improvements to the cleaning process, so that immature seeds are eliminated before they go into storage. If seeds were ungerminated but still alive, perhaps there was something missing from the physical environment provided by the germination tests. Cut tests help you discover how to make improvements to your seed quality management.
Dormancy
Let’s assume you have ensured optimum substrate, temperature, gaseous environment and water for your seeds. You have established that the seeds are not immature, dead or empty. But still they have not germinated. Does that mean they are not viable? Not necessarily! The seeds may be dormant.
Dormant seeds are operating on minimum metabolism, so even when environmental conditions are adequate, something else needs to happen to break the dormancy. In the wild, plants that have evolved dormancy have done so because it is useful to their survival. Sometimes dormancy allows a plant to spread germination over time and space. Sometimes it allows the plant to synchronise its germination with optimal conditions for growth.
In genebanks, too, dormancy can be useful. It can help maintain the quality of seeds in storage. It can protect seeds from sprouting in wet and moist conditions before they enter the genebank. It can prevent seeds from germinating under unfavorable conditions, such as inside the active and base stores. It can help the seeds to remain alive for many years, ensuring a continuous source of new plants.
However, unlike in situ ecosystems, genebanks cannot rely on the rhythms of nature to break dormancy. So if you are working in an ex situ collection, a thorough understanding of the types and mechanisms of dormancy is essential, to help you work out how to coax seeds into germination.
Pause for reflection
Dormancy is less common in crops than wild relatives, because over millennia of cultivation by humans, the tendency was for dormancy to be selected against. In the past, farmers tended to select types of seeds that germinated readily when sown, even though this desirable habit may be linked to less desirable ones, such as sprouting too readily in storage, resulting in wasted seed.
Paradoxically, in the twenty-first century, re-introducing a limited amount of dormancy could be helpful to farmers who are growing crops in locations where they are prone to pre-harvest sprouting. It could eliminate this well-documented cause of waste.
Take five minutes to think about how wild relatives conserved in genebanks could be a useful source of desirable traits related to dormancy.
Breaking dormancy in genebanks
Watch this video, in which scientists and genebank managers introduce dormancy strategies used by plants in the wild, and some of the measures that genebanks have adopted to break dormancy in the laboratory. The video mentions two different types of dormancy (physical dormancy and physiological dormancy). You will learn more about these later.
As you watch the video, think about how environmental conditions make dormancy a good strategy for seeds in nature, and how scientists at genebanks can mimic aspects of the natural world in order to give dormant seeds the best possible chance to germinate.
Transcript: Video 2: Breaking dormancy
Please use the box to write your comments on what the scientists say. What dormancy strategies do plants adopt in the wild? What conditions in situ are they adapted for? And how do laboratory techniques set out to mimic these conditions in the genebank? You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
Fiona Hay explains that dormancy is a strategy used by plants to spread the germination of seeds over space and time.
One example of spreading germination over space is legumes with a thick coat. An animal eats the seeds, and the seed coat is abraded, but not fully digested, inside the animal’s digestive tract. This breaks the dormancy. Meanwhile, the animal may have moved a long distance from the parent plant before excreting the seeds, now germination-ready. To overcome this kind of dormancy in the lab, scientists may use a scalpel to break the seed coat.
One example of seeds spreading germination over time is found in climates where one season is more conducive to germination than others. In temperate climates, dormancy allows seeds to delay germination over the winter, and to germinate in spring when conditions are better. To overcome this kind of dormancy in the lab, scientists may use either temperature changes or chemicals that resemble the plant hormones that would naturally be produced in the spring.
Changes in dormancy over time
Seeds can go in and out of dormancy at different stages of their existence. Primary dormancy can be found in seeds dispersed directly from the mother plant. It develops as a result of an interaction between the genotype of the seed and the environmental conditions surrounding it.
Seeds arriving at a genebank with primary dormancy can mature in the drying room, so they are no longer dormant by the time they are packed and stored. By contrast, seeds arriving at a genebank in a non-dormant state may move into a dormant state under the conditions in the genebank. For example, a seed that is viable, but not given enough water to allow imbibition, may develop a type of induced dormancy known as secondary dormancy. This brings advantages in the wild, since it can protect a seed from germinating during a drought.
The reverse can also happen. Seeds in a dormant state may transition into non-dormancy in a process called after-ripening. Some seeds in the population gradually become more responsive to conditions that promote germination, and less responsive to conditions that restrict it. Rice and wild oats are examples of seeds that after-ripen in warm, dry conditions – in the video, you saw rice being given an after-ripening treatment by warming it in the oven.
As a result of the release of primary dormancy and the induction of secondary dormancy, it is quite possible for a seed lot that has been in storage for some time to contain seeds at different stages along the continuum of levels of dormancy. This is why, when studying dormancy, it is a good idea to use fresh seeds.
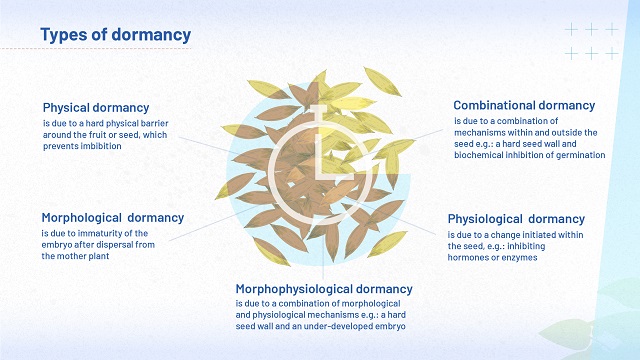
Types of dormancy
Seeds in situ face a variety of environmental challenges, and the mechanisms they have evolved in response to these challenges show a lot of diversity. There are three main types of dormancy - all of them maximise the chances of survival for a particular seed in its particular setting:
- Physiological dormancy is a type of dormancy initiated from within the seed.
- Morphological dormancy prevents germination because the embryo is under-developed after the seed has been removed from the mother plant.
- Physical dormancy is a type of dormancy initiated by a hard physical barrier around the fruit or seed, which prevents water from entering the seed.
Some seeds may exhibit more than one of these types of dormancy:
- Combinational dormancy is found in seeds with both physiological and physical dormancy.
- Morphophysiological dormancy is found in species that have both physiological dormancy and immature embryos that need time to grow.
In the next few pages, you will discover more about these types of dormancy, and why successful germination sometimes requires a lot more than just temperature, substrate and water.
Physiological dormancy
Physiological dormancy is initiated by the embryo or surrounding tissues. The mechanism may be the presence of hormones or enzymes inside the seed, which prevent germination. Here are a few examples:
In forests of Africa, shade-intolerant plants stand a better chance of germination when the dense, tall vegetation of the upper storey is cleared by fire, creating a temporary gap in the canopy for light to flood in. Physiological dormancy allows some seeds to wait on the forest floor until the dormancy is broken by one of the chemicals in smoke.
In woodlands of Northern Europe, the pattern of cold winters and warm summers means that spring is the best time for some seeds to germinate: those seeds may go into dormancy during the cold months of winter. This type of physiological dormancy is broken by warmer temperatures of spring.
In the sand dunes of Western Australia, seeds stand the best chance of germination when buried deep underground, where the sand is more stable, and oxygen is present: for instance the dune-stabilizing rhizomatous grass Spinifex hirsutus will not germinate unless it is dark and there is oxygen available.
Morphological dormancy
Morphological dormancy is a type of dormancy linked to the development of the seed. It prevents germination because the embryo is under-developed, even after the seed has dispersed from the mother plant. After natural dispersal, the embryo still needs a period of time to develop before it is able to germinate.
Case study: wood anemone

In European woodlands between March and May, the star-shaped flowers of wood anemone, Anemonoides nemorosa, emerge among the leaf litter of ancient forests. Wood anemone is of conservation interest in the United Kingdom, and its seeds are conserved at the Millennium Seed Bank at Wakehurst, part of the Royal Botanic Gardens, Kew. However, when these seeds were retrieved from storage, germination was poor.
A team of scientists at Kew suggested that the low germination rates could be the result of morphological dormancy. The seeds of Anemonoides nemorosa are immature at the point of natural release: could this make them unable to survive a period of desiccation in storage? When the scientists buried the seed-bearing fruits in the leaf litter of their native woodland habitats, or left them to develop under laboratory conditions prior to storage, this allowed time for the embryos to develop from globular, to heart-shaped, to torpedo-shaped embryos, as Figure 8 (below) shows. This intervention improved germination rates after storage significantly.
Physical dormancy
Unlike physiological dormancy, which has its mechanisms inside the seed, physical dormancy is initiated by a mechanism outside the seed. Only about fifteen plant families (including Anacardiaceae, Cannaceae, Convolvulaceae, Fabaceae, and Malvaceae) exhibit physical dormancy. The mechanism for this type of dormancy is a hard physical barrier, which surrounds either the fruit or the seed. This prevents water from entering the seed, and therefore prevents imbibition. Inside this physical barrier, seeds can survive for a long time, but something else needs to happen in order to break the dormancy. Here are some examples:
In fire-prone areas of Australia, some species from the genus Banksia keep their seeds inside a woody fruit for years. During a forest fire, the heat of the fire will open up the fruit, which breaks the dormancy of the seeds inside. They fall to the ground, where, once the fire has passed through, conditions are optimal for the seeds to germinate.
In semi-arid areas of Central America, the legume Stylosanthes humilis has seeds with water-impermeable coats and a pore, called a water gap, which closes at seed maturity. The water gap opens in response to fluctuations in temperature, which breaks the dormancy by enabling the seed to take in water and oxygen.
Case study: Acacias and giraffes
South Africa’s Acacia burkeii is a legume whose seeds have hard seed coats. The physical dormancy this gives them is important for survival. The process starts when a giraffe eats acacia seeds. As the seeds pass through the animal’s digestive tract, the acidic conditions, microorganisms and enzymes in the animal’s gut have the effect of scarifying their hard seed coats. Meanwhile, the giraffe may travel far from the parent plant, before depositing the scarified seeds in feces. This not only breaks dormancy, but also distributes the seeds a long way from the parent plant, reducing the risk of competition between young seedlings and the parent plants.
Combinations of dormancy types
Some species can show a combination of different types of dormancy, leading to a complex layering of dormancy mechanisms. It may take careful detective work to establish the best way to germinate such seeds. In order for germination to occur effectively, all types of dormancy must be overcome. For instance, under genebank conditions, germination rates for the Western Australian shrub Diplopeltis huegelii are highest when a hot water treatment is used to break physical dormancy, followed by a thirteen-month period of maturation to break morphological dormancy.
Figure 10 (below) is a reminder of the most common causes of dormancy: You might like to view and download this image at high quality. To do this, first click the 'Maximise' link below the image: to download, right click or control click once the image has opened.
Reviewing your progress
Congratulations on getting this far in the course! There is more studying to do, but now is a good time to step back, pat yourself on the back, and make sure you’re prepared for the end of the course.
After finishing module 5, you will be invited to do an end-of-course quiz. This is one of the requirements you need to have passed in order to earn a digital badge and certificate of participation. You also need to have worked through the entire course, and participated in all discussions. So now is a good time to go back and check that you have read every page of text and posted in every discussion.
Feedback
We would very much like you to give us feedback on the course. This is your opportunity to tell us what you enjoyed in the course, and what we can do better. We will use your feedback to plan the experience of future learners.
Dormancy breaking treatments
If you suspect seeds are not germinating because of dormancy, it is important to find out as much information as you can about the species. Conduct a literature search on its behavior in storage, and climate data for the conditions it grows under in situ. Then consider which dormancy-breaking treatments might be relevant, from the list of treatments below.
Treatments for dormancy:
Scarification: is a process of surgically breaking the seed coat. In some seeds scarification is thought to mimic the way the seed coat decays as a result of diurnal temperature fluctuations or microbial activity. In other seeds, it may mimic digestion by animals or the heat of fire. If your seeds do not come from the families known to exhibit physical dormancy, you do not need to scarify.
Stratification: is a process of altering the temperature to simulate changing seasons. Depending where the seed originates, stratification can mimic the cold of winter, or the heat of a dry season.
Dry after-ripening: is a process of drying, which is thought to destroy hormones that suppress germination.
Chemicals: gibberellic acid (GA3) may tilt the seed’s biochemistry in a direction more favorable to germination by stimulating enzymes that convert starch into sugar.
Dark and light: modifying light conditions may exert dormancy-breaking effects by gradually converting the pigment phytochrome R into phytochrome FR, or altering the activity of hormones and enzymes.
Time: can break morphological dormancy by enabling the seed to develop under suitable ambient conditions for them to mature.
Activity 2
Allow five minutes for this activity.
For each of the treatments above, think about which type of dormancy (i.e.: physical, morphological, physiological) is most likely to respond positively to that treatment. Write your answers in the note-writing box before looking at the authors’ comments.
When you are ready, press 'reveal' to see our comments.
Discussion
You probably made connections between scarification and physical dormancy, time to mature and morphological dormancy, and chemical treatments with physiological dormancy. It’s worth bearing in mind that some treatments can be effective for a variety of different types of dormancy, for instance scarification can be used to overcome both physical and physiological dormancy. Where a species shows more than one type of dormancy, you may need to try different treatments.
My genebank and me
For your next blog entry, think about any seeds that you have encountered in your genebank, which were difficult to germinate. Could they have been dormant? How did your genebank resolve the issue? Is there anything you would do differently now that you have done this course?
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
The seed information database
Throughout this course you have used the Seed Information Database to find out about the behavior of seeds under various conditions. You should by now be familiar with how to interrogate this useful resource.
Activity 3: exploring the Seed Information Database
Now go back to the search tool of the Seed Information Database. Look up a variety of cowpea called Vigna unguiculata spontanea. How does the database suggest you should break its dormancy prior to sowing? Now see what you can find out about any seeds in your own genebank that you think might exhibit dormancy. The Seed Information Database is a good starting point, but you might need to use other articles or resources to find out more about your chosen species.
Use the text box below to record what you have learned. These notes are for your own development: they will not be shared with course colleagues or moderators.
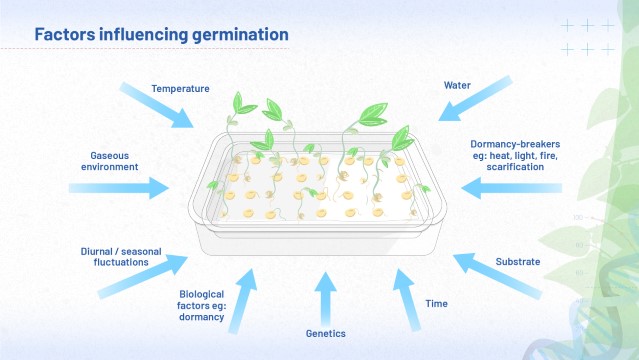
Factors influencing germination
Throughout module 5, we have explored various factors which can affect whether a seed will germinate or not. Figure 11 reminds you of these. You might like to view and download this image at high quality. To do this, first click the 'Maximise' link below the image: to download, right click or control click once the image has opened.
What happens if you have ensured optimum substrate, temperature, gaseous environment and water for your seeds to germinate, established that none of your seeds are immature, dead or empty, and applied the appropriate dormancy-blocking treatments, but still the germination rates are disappointing for the species? At this point, it may well be time to regenerate.
Regeneration
In Module 1, you learned that maintaining the genetic integrity of seeds is a core objective of genebanks. Many of the processes in subsequent modules are geared towards maintaining this genetic integrity. Whether by drying seeds to maximize their longevity or by cross-checking newly regenerated seeds against the seed file during cleaning, there are many ways a genebank can improve the quality of regenerated seeds. This make sure that the new generation of accessions are exactly the same, or at least as close as possible, to the original sample.
Genetic integrity can be threatened by pollen contamination, seed contamination, or unintended selection over many cycles of regeneration. Watch the video in which scientists talk about what measures they take to reduce genetic drift in regenerated seeds. As you watch, think about how every process tightly tied into the genebanks’ core mission of conserving diversity.

Transcript: Video 3: seeds for the future
Please write your comments on what Andres Godwin Sajise, Marionette Alana and Fiona Hay say. What measures do they use to avoid genetic drift? Why does ensuring seed quality at earlier stages of the process, such as harvesting, drying and storage, also help reduce genetic drift? You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
The video shows various ways in which genebanks avoid genetic drift during regeneration. By checking the database, they ensure that they only embark on regeneration on accessions for which there is evidence that it really is needed. By planning when and where to plant, they reduce the risk of cross-fertilization or contamination during flowering or harvest. And by only regenerating a limited number of times, they increase the likelihood that new generations are as close as possible to the original sample. Most of all, by applying the science of seed quality during the earlier stages of cultivation, harvesting, drying, sorting and storage, genebanks can increase the amount of time between regeneration cycles.
The FAO’s Genebank Standards
The conditions laid out in the FAO’s Genebank Standards are a guide to storage. They do not provide procedural detail on germination testing, dormancy breaking, or how these can feed into decisions about when to regenerate. However, they do provide guidance on how often testing should happen.
- 4.3.1 The initial seed viability test should be conducted after cleaning and drying the accession, or at the latest, within 12 months after receipt of the sample at the genebank.
- 4.3.2 The initial germination value should exceed 85% for most seeds of cultivated crop species. For some specific accessions and wild or forest species that do not normally reach high levels of germination, a lower percentage could be accepted.
- 4.4.1 The most original sample should be used to regenerate accessions.
- 4.4.1 Regeneration should be carried out in such a manner that the genetic integrity of the accession is maintained. Species-specific regeneration measures should be taken to prevent admixtures of genetic contamination.
- 4.4.3 If possible at least 50 seeds of the original and subsequent most-original sample should be archived in long-term storage for reference purposes.
Discussion point: germination and dormancy
This is the final discussion point of the course.
Participation in this and the other discussions within the course is essential for you to gain your end-of-module badge and completion certificate.
You should use these discussions to:
- Explore common dilemmas in seed quality management
- Listen to the opinions of other learners and the facilitator
- Come to a collective view on how to proceed when faced with this type of scenario in your own work
Discussion space: germination and dormancy
What can genebanks do to improve germination rates for seeds in medium and long-term storage?
Discussion spaces:
As you move through the course, you may wish to return to the discussion spaces to add further posts or to read what other learners have posted.
You can access the discussion spaces from the main menu on the left-hand side of this page.
Key words and concepts

Throughout this course, we offer you quizzes to help you keep track of how much you have learned. We hope you find them enjoyable. The quizzes within modules are not graded, and your results will not be shared with colleagues, but engaging with these quizzes will help you develop your own understanding. It is important to check your answers and read the feedback we have written: this feedback is often the best way to learn.
Working through the quizzes in every module will build your confidence until by the end of the course, you will be ready to tackle the end-of-course quiz. Unlike the quizzes within modules, this final quiz will count towards your badge and statement of participation.
Have a go at this quiz, testing your understanding of key ideas in module 5.
Summary
You have now reached the end of the online learning component of this course. In this final module, you have deepened your understanding of the conditions seeds need to germinate. You have discovered what dormancy is, and observed some common techniques that are used to break dormancy.
During this course, you have read the latest scientific research about genebanks’ practice. You have watched videos of international genebanks tackling matters of seed quality. You have made connections between the strategic objectives of genebanks (such as seed longevity, viability and genetic integrity) and the day-to-day procedures carried out in order to meet those objectives. Having studied this course, you should not only have a better appreciation of how to manage seed quality, but also the scientific reasons why these techniques work.
You might like to download the notes you have made during the course and take them away with you after you have finished. You do this by clicking 'Download your answers for the documents on this course', as shown in the illustration below. This will generate a .pdf file that you can download.

Even after the course is finished, you will still be able to refer back to it. You can still log in to review the main text with its useful resources and links to external websites and documents. The videos and animations will still be at your disposal. So will the additional reading in 'Useful publications'.
Remember, in order to gain your digital badge and certificate of completion, you need to have worked through the entire course, and participated in all discussions. If you are sure you have done this, it is time to proceed to the end of course quiz. As well as proving to others that you have engaged successfully with the course, this final quiz will help you to consolidate your learning.
Module 5 at a glance:
- Germination tests provide crucial information about the longevity and viability of seeds in storage.
- To be sure the results of a germination test are reliable, ensure that you provide all the conditions a seed needs to germinate (including but not limited to: water, substrate, oxygen and temperature).
- Dormancy is more common in wild species than crops, since it has been selected against in crops.
- There are different types of dormancy: primary or secondary; physical, physiological or morphological, or combinations of these.
- The different types of dormancy have different mechanisms, and their onset can be at different times in a seed’s life in storage.
- Dormancy can be broken by dormancy-breaking techniques, which vary according to species: the most common of these are scarification and manipulating temperature.
Useful publications
If you were interested in some of the issues in this module, you might like to download and read these articles, which we have selected for you.
Al-Amery, M., Geneve, R., Mauricio, F. Sanches M., Armstrong, P., Maghirang, E., Lee, C., Viera, R., Hildebrand, D., (2018). Near-infrared spectroscopy used to predict soybean seed germination and vigour. Seed Science Research, 28, special issue 3, pp. 245-252.
Available at: https://doi.org/ 10.1017/ S0960258518000119.
Ali, N., Probert, R., Hay, F., Davies, H., Stuppy, W., (2007). Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research, 17, pp. 155-163.
Available at: https://doi.org/ 10.1017/ S0960258507783149.
Baskin J and Baskin. (1998). Seeds: ecology, biogeography and evolution and dormancy. Cambridge, Mass: Elsevier.
Available at: https://doi.org/ 10.1016/ B978-0-12-080260-9.X5000-3.
Feng, J., Shen, Y., Shi, F., Li, C., (2018). Embryo Development, Seed Germination, and the Kind of Dormancy of Ginkgo biloba L. Forests , 9(11), 700.
Available at: https://doi.org/ 10.3390/ f9110700.
Gao, F., Ayele, B. (2014). Functional genomics of seed dormancy in wheat: advances and prospects. Frontiers of Plant Science, Sept 15, 5, 458.
Available at: https://doi.org/ 10.3389/ fpls.2014.00458.
Hay, F. R., Whitehouse, K. J. (2017). Rethinking the approach to viability monitoring in genebanks. Conservation Physiology, 5 (1)
Available at: https://doi.org/ 10.1093/ conphys/ cox009.
ISTA (2024). International rules for seed testing. International Seed Testing Association, Bassersdorf, Switzerland
Available at: https://www.seedtest.org/ en/ publications/ international-rules-seed-testing.html.
Joosen, R., Kodde, J., Willems, L., Ligerink, W., van der Plas, L., Hillhorst, H. (2010). GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. The Plant Journal, 62, 1
Available at: https://doi.org/ 10.1111/ j.1365-313X.2009.04116.x.
Jaganathan, G. (2020). Defining correct dormancy class matters: morphological and morphophysiological dormancy in Arecaceae. Annals of Forest Science, 77, 100.
Available at: https://doi.org/ 10.1007/ s13595-020-01010-7.
Nehoshtan, Y., Carmon, E., Yaniv, O., Ayal, S., Rotem, O. (2021). Robust seed germination prediction using deep learning and RGB image data.Scientific Reports, 11, 22030.
Available at: https://doi.org/ 10.1038/ s41598-021-01712-6.
Pausas, J., Lamont, B., (2022). Fire-released seed dormancy - a global synthesis. Biological Reviews, 97 (4) pp. 1612-1639
Available at: https://doi.org/ 10.1111/ brv.12855.
Ray, S., Vijayan, J., Sarkar, R. (2016). Ray, S., Vijayan, J., Sarkar, R. (2016). Frontiers in Plant Science, May, 18: 7:671
Available at: https://doi.org10.3389/ fpls.2016.00671.
Venier, P., Carrizo García, C., Cabido, M., Funes, G., (2012). Survival and germination of three hard-seeded Acacia species after simulated cattle ingestion: The importance of the seed coat structure. South African Journal of Botany, 79, pp. 19-24.
Available at: https://doi.org/ 10.1016/ j.sajb.2011.11.005.