Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 4 December 2025, 5:05 AM
Parkinson's: Managing bone health and fracture risk
1 Falls in Parkinson’s
The following video provides you with a brief overview of this course about managing bone health and fracture risk in Parkinson’s, and the content of the course.
Transcript
Hello and welcome to the Parkinson's bone course. I'm Dr Veronica Lyell. I’m a consultant geriatrician with a specialist interest in Parkinson’s and Bone Health. My consultant colleagues, Dr Emily Henderson and Dr Celia Gregson, and I, here in the Royal United Hospital Bath, have developed this course in conjunction with Parkinson's UK and the Open University.
You will be very familiar with falls in Parkinson's. Falls can have many serious consequences, including injuries, hospital admission, and loss of confidence, and one particular fear is that of hip fracture.
Hip fractures cause great pain and loss of function. They’re compounded with complications such as pneumonia, delirium, and pressure sores. People with Parkinson’s are more likely to suffer these complications. Not only that, but people with Parkinson’s are more likely to sustain a hip fracture in the first place; indeed, a man with Parkinson’s has three times the chance of breaking his hip compared with another man of the same age.
There are real opportunities to intervene for our patients with Parkinson’s to reduce the burdens of fractures and we hope that this course will empower you to take these opportunities.
It is designed to provide an overview of falls assessment, to explain bone metabolism and osteoporosis, to introduce concepts of fracture risk assessment and to give management options, from lifestyle interventions through to drug therapies. There are modules to work through, each with interactive features that we hope will aid your understanding and learning.
By the end of the course we hope you will have developed the confidence to tackle bone health assessment and management in Parkinson’s. People with Parkinson’s have enough to contend with without fractures, and we are passionate about helping them reduce the risk of broken bones.
In section 1, you will gain:
- an overview of the epidemiology of falls in Parkinson’s
- an understanding of why people with Parkinson’s fall
- an awareness of the potential sequelae of falls
Throughout this course we will return to this case study to inform learning
CASE STUDY
Mr Smith is a 74 year old man, diagnosed with idiopathic Parkinson’s, who has been seen in clinic for the last 8 years. He is taking sinemet 125mg four times a day. He was diagnosed with prostate cancer last year and is now on anti-androgen hormone treatment. He had polymyalgia rheumatica 3 years ago and had steroid treatment for 18 months. He has some back pain. He drinks 3 units of red wine daily. He suffers with constipation which is easily managed with aperients. He is hypothyroid on monitored replacement therapy. He takes bendroflumethiazide for hypertension, zopiclone to aid sleep as well as aspirin 75mg for secondary cardiovascular disease prevention. He comes to clinic and it is noted that he is shuffling more than he used to and now uses a stick to walk. Coming through the door he gets ‘stuck’ and freezes. There is no ‘wearing off’ phenomenon. On examination he is wearing bifocal glasses, but his eye movements are normal. He has Hoehn and Yahr stage 3 Parkinson’s and has a moderately stooped posture. He has moderate bradykinesia and rigidity of his upper and lower limbs, more evident on the left than the right and no red flags to suggest an alternative diagnosis to idiopathic Parkinson’s. He weighs 57kg and is 1.59m tall. He reports his main problem as feeling ‘really unsteady’ and as a result he is less confident leaving the house to do his weekly shop. He has not fallen.
1.1 Who falls with Parkinson’s?
Falls are a common and devastating complication of Parkinson’s. A meta-analysis of six independent, prospective studies that examined the incidence of falls in people with Parkinson’s demonstrated that 46% (95%CI 38-54%) fell over 3 months (Pickering et al, 2007). In patients who had not previously fallen, this rate was still high at 21% (95%CI 12-35%). In a prospective cohort study based in Sydney, 87% of the patients who remained alive at 20 years post diagnosis, had experienced a fall (Hely et al, 1999). A more recent meta-analysis has demonstrated that 60% of people with Parkinson’s fall annually and 39% fall recurrently (Allen et al, 2013). In recurrent fallers median survival is reduced to 6 years (Wenning et al, 1999).
The rate of falls increases as the condition progresses, reaching a peak at Hoehn and Yahr stage 3 (Balash et al, 2005). At this point, people with Parkinson’s are moderately impaired with reduced stability – but still mobile. Falls are more common in older people with Parkinson’s. Evidence from the ICICLE cohort which comprises people with newly diagnosed Parkinson’s, supports the fact a falls can occur early on in the course of disease, and that, once occurred, falls become recurrent (Hunter et al, (2018):
| Stage | Hoehn and Yahr Scale |
| 1 | Unilateral involvement only usually with minimal or no functional disability |
| 2 | Bilateral or midline involvement without impairment of balance |
| 3 | Bilateral disease: mild to moderate disability with impaired postural reflexes; physically independent |
| 4 | Severely disabling disease; still able to walk or stand unassisted |
| 5 | Confinement to bed or wheelchair unless aided |
1.2 Why do people with Parkinson’s fall?
As with most older people who experience a fall, the majority of falls in Parkinson’s result from the interplay of a lot of factors. Some of these are generic (that is, they may affect people without Parkinson’s) whereas some are specific to, or much more common in, Parkinson’s.
Reflective exercise 1
Use your reflective log to draw a table similar to the following:
| Generic | Parkinson’s specific |
|
Then from the following list of risk factors for falls, place each factor into the correct column of your table:
| text list | text list |
|---|---|
| anticholinergics for tremor or bladder | age |
| antidepressant medication | anxiety |
| nocturia | history of previous falls |
| axial rigidity | high doses of levodopa |
| postural hypotension | difficulty with performing dual tasks |
| sedative medication | poor balance |
| inappropriate polypharmacy | cardiac arrhythmia |
| freezing of gait | abnormal posture |
| dyskinesia | muscle weakness |
| arthritis and joint problems | visual impairment |
| daily drinking of alcohol | environmental hazards |
| peripheral neuropathy | cognitive impairment |
Discussion
Your table should look like this:
| GENERIC | More common in or specific to Parkinson’s |
| history of previous falls | high doses of levodopa |
| age | anticholinergics for tremor or bladder |
| antidepressant medication | postural hypotension |
| inappropriate polypharmacy | freezing of gait |
| sedative medication | abnormal posture |
| cardiac arrhythmia | poor balance |
| arthritis and joint problems | nocturia |
| anxiety | difficulty with performing dual tasks |
| muscle weakness | axial rigidity |
| visual impairment | dyskinesia |
| daily drinking of alcohol | |
| environmental hazards | |
| peripheral neuropathy |
Parkinson’s is associated with distinct abnormalities in balance and gait.
Balance abnormalities include:
- abnormal posture
- increased sway
- inability to change sensory weighting
- reduced limit of stability
- altered postural strategies to perturbation
- abnormal anticipatory adjustments
It can be useful to classify gait abnormalities into two types. The first affects one footstep to the next, including:
- slower, more irregular walking
- asymmetry between left and right footsteps
The second type occurs episodically and can include:
- freezing episodes, in which patients describe their feet as being stuck to the floor like glue
- festination – whereby increasingly short steps are taken as the feet ‘run away’ with themselves
The intermittent, episodic and often unpredictable nature of gait freezing is a very strong risk factor for falling.
Medication and falls
Being on lots of prescribed medication (polypharmacy) is itself associated with an increased risk of falls. But some medications bring higher risks than others, probably through their effects on balance and cognition. Parkinson’s-specific medication can improve falls risk if it improves gait and balance, but it can also be associated with falls because of its potential negative effects on cognition and blood pressure.
Please complete the following quiz. This is formative. It is really helpful in consolidating your learning, but there is no pass mark:
1.3 What are the consequences of falls?
Falls are devastating. They have a direct impact on the person affected, their family and caregivers, as well as incurring considerable economic cost.
A quarter of all falls result in injury. Injuries range from soft tissue injuries, through to fractures and head injuries that require hospitalisation. Even before Parkinson’s has been diagnosed the risk of sustaining a fracture and traumatic brain injury is increased compared to those without Parkinson’s (Camacho-Soto et al, 2017). This may suggest that there are sub-clinical motor and non-motor features present that contribute to this risk.
People with Parkinson’s are at higher risk of experiencing traumatic brain injury following a fall. Fracture risk is doubled in Parkinson’s with a tripled risk of hip fracture (Pouwels et al, 2013). Once a hip fracture has occurred, people with Parkinson’s are at higher risk of sustaining a second hip fracture (Harvey 2018). Vertebral fractures are also common but the associated stooped posture may be wrongly attributed to Parkinson’s alone (Torsney et al, 2014). Hip fractures are sustained more commonly than wrist fractures as people with Parkinson’s tend to fall ‘like a log’ with their arms adducted to their sides (Carpenter et al, 2004). In recent years, hip fractures have accounted for 4% of all emergency admissions in Parkinson’s and for 7.6% of admission costs (£13 million per annum for England and Wales in 2014) (Low et al, 2015).
Hip fractures may have grave consequences, with only half of patients returning to their previous level of function (Holt et al, 2008). Even when we take into account poorer pre-fracture mobility, the consequences of hip fractures for people with Parkinson’s are worse than among the general population. This is because they experience higher pressure sore rates, a more than doubled length of stay in hospital and a greater proportion of bed/wheelchair dependency after 30 days (Walker et al, 2013). Parkinson’s-specific institutionalisation and mortality rates after hip fracture are unknown but are likely greater than the general population.
As a result of falling, many people with Parkinson’s develop ‘fear of falling’. This concept encompasses concerns about falling and loss of balance confidence: It also includes low fall-related efficacy, which is someone’s confidence or belief in their ability to perform activities without losing balance or falling. This can lead to activity avoidance. In turn, people who are fearful of falling restrict their social activities and limit their daily activity. This results in loss of independence and isolation, which in turn contributes to immobilisation and perpetuates a cycle of deterioration.
Falls tend to occur in parallel with the emergence of other debilitating, difficult-to-treat features of the condition. This includes dysphagia and cognitive impairment. These features, along with fear of falling, contribute to a state in which people suffer the consequences of immobility. This includes pressure sores, constipation, deconditioning, osteoporosis, inactivity and loss of independence, which in turn contribute to a vicious cycle that further increases the risk of falls.
This section has illustrated the dangers of falls. The next section will look at how to reduce falls risk.
1.4 Management of patients who are at risk of falls
Falls are a major risk for many older people, but there is good evidence that the risks of falls and fractures can be reduced with a multifactorial approach. In order to tackle fracture risk in Parkinson’s, it is essential that a falls risk assessment is carried out.
In clinic, all patients should be asked about previous falls as this is a strong predictor of having a fall in the future. Identifying episodes of near falls is also important as they may precede/indicate the occurrence of future falls.
A thorough and systematic search should be performed to identify any modifiable factors that led to the patient falling and these should be proactively tackled (van der Marck et al, 2014). Interventions will include:
- optimisation of medication strategy
- programmes of physiotherapy or balance classes
- other generic factors such as visual assessment and modification of environmental factors
Further details of how these risk factors are tackled are beyond the scope of this section but readers are directed to Parkinson’s and falls: tips & resources from the Parkinson’s West Midlands Excellence Network [link please], which includes guidance and further resources.
All patients should have their bone health assessed and actively treated.
The approach to this is detailed in Fracture risk assessment in Section 3 and Treatment options for osteoporosis in Parkinson’s in Section 4.
1.5 Reflective exercise: Falls risk in Parkinson’s
Read over Mr Smith’s history. On direct questioning he does admit to having had one fall.
Think about what you have learned in Section 1 regarding factors which make someone with Parkinson’s more likely to fall. Remember that falls risk can be increased both by Parkinson’s factors and other more general factors. There are several things mentioned in the text which may increase Mr Smith’s falls risk. In your reflective log, identify as many as you can, and suggest how to reduce their impact. Write down anything else you would like to check.
Mr Smith is a 74 year old man diagnosed with idiopathic Parkinson’s who has been seen in clinic for the last 8 years. He is taking sinemet 125mg four times a day. He was diagnosed with prostate cancer last year and is now on anti-androgen hormone treatment. He had polymyalgia rheumatica 3 years ago and had steroid treatment for 18 months. He has some back pain. He drinks 3 units of red wine daily. He suffers with constipation, which is easily managed with aperients. He is hypothyroid on monitored replacement therapy. He takes bendroflumethiazide for hypertension, zopiclone to aid sleep, as well as aspirin 75mg for secondary cardiovascular disease prevention. He comes to clinic and it is noted that he is shuffling more than he used to and now uses a stick to walk. Coming through the door he gets ‘stuck’ and freezes. There is no ‘wearing off’ phenomenon. On examination he is wearing bifocal glasses, but his eye movements are normal. He has Hoehn and Yahr stage 3 Parkinson’s and has a moderately stooped posture. He has moderate bradykinesia and rigidity of his upper and lower limbs, more evident on the left than the right, and no red flags to suggest an alternative diagnosis to idiopathic Parkinson’s. He weighs 57kg and is 1.59m tall. He reports his main problem as feeling ‘really unsteady’ and as a result he is less confident leaving the house to do his weekly shop. He has not fallen.
Discussion
You may have noted the following factors:
- He is 74, and falls grow more common with age.
- He has Parkinson’s, which affects balance and gait.
- He is on dopaminergic medication. So while adequate treatment is necessary to maximise motor control, you would want to consider whether the drugs were negatively affecting his cognition and if they were making him dyskinetic or contributing to postural hypotension.
- Other medications associated with falls are sedatives (zopiclone) and antihypertensives (bendroflumethiazide) as well as alcohol intake.
- He demonstrates clear gait abnormality (shuffling steps) and freezing of gait, which is a potent risk factor for falling.
- His posture is abnormal (stooped) and he reports being unsteady.
- Bifocal glasses are an established risk factor for falls, as the magnifying section distorts the image in the lower field of vision.
1.6 Summary
In this section, you will have gained an overview of the epidemiology of falls in Parkinson’s – understanding that falls are a frequent and very debilitating consequence of the condition. You will have gained insight into the specific consequences of falls including fractures, soft tissue injury and fear of falling. We presented an overview of the reasons why people with Parkinson’s fall, dividing these into those that are generic risk factors and those that are specific to Parkinson’s. We also touched on approaches to reduce the risk of falls.
You have now completed section 1. Section 2 looks in more detail at bone metabolism and structure.
1.7 Useful links
1.8 References
| Section and author/date | Reference |
| 1.1 Pickering et al (2007) | Pickering RM et al (2007) ‘A meta-analysis of six prospective studies of falling in Parkinson’s disease’ Movement Disorders; 22:189–900 |
| 1.1 Hely et al (1999) | Hely MAM et al (1999) ‘The Sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years’ Journal of Neurology, Neurosurgery and Psychiatry; 67:300–307 |
| 1.1 Allen et al (1999) | Allen NE et al (2013) ‘Recurrent falls in Parkinson’s disease: a systematic review’ Parkinson’s Disease; 2013 |
| 1.1 Wenning et al (1999) | Wenning GK et al ‘Progression of falls in post-mortem-confirmed Parkinsonian disorders’ Movement Disorders; 14:947–950 |
| 1.1 Balash et al (2005) | Balash Y et al (2005) ‘Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors’ Journal of Neurology; 252:1310–1315 |
| 1.1 Hunter et al (2018) | Hunter et al (2018) ‘Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition’. Disability and rehabilitation. 11;40(19):2236-41. |
| 1.3 Camacho-Soto et al (2017) | Camacho-Soto et al (2017) ‘Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using Medi- care data’. Ann Neurol; 82:744–754. |
| 1.3 Pouwels et al (2013) | Pouwels S et al (2013) ‘ Risk of fracture in patients with Parkinson’s disease’ Osteoporosis International; 24:2283–90 |
| 1.3 Harvey et al (2018) | Harvey et al (2018) ‘Incidence, timing and impact of comorbidity on second hip fracture: a population‐based study.’ ANZ Journal of Surgery, 88: 577-581. |
| 1.3 Torsney et al (2014) | Torsney KM et al (2014) ‘Bone health in Parkinson’s disease: a systematic review and meta-analysis’ Journal of Neurology, Neurosurgery and Psychiatry; 85: 1159–1166 |
| 1.3 Carpenter et al (2004) | Carpenter MG et al (2004) ‘Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease’ Journal of Neurology, Neurosurgery and Psychiatry; 75:1245–54 |
| 1.3 Low et al 2015 | Low V et al ‘Measuring the burden and mortality of hospitalisation in Parkinson’s disease: a cross-sectional analysis of the English Hospital Episodes Statistics database 2009–2013’ Parkinsonism Related Disorders; 21: 449–454 |
| 1.3 Mühlenfeld et al (2019) | Mühlenfeld et al (2019). Fractures in Parkinson’s Disease: injury patterns, hospitalization, and therapeutic aspects. Eur J Trauma Emerg Surg. https://doi.org/10.1007/s00068-019-01240-z |
| 1.3 Holt et al (2008) | Holt G et al (2008) ‘Outcome after surgery for the treatment of hip fracture in the extremely elderly’ The Journal of Bone and Joint Surgery; 90: 1899–1905 |
| 1.3 Walker et al (2013) | Walker RW et al (2013) ‘Hip fractures in people with idiopathic Parkinson’s disease: incidence and outcomes’ Movement Disorders; 28:334–340 |
| 1.3 Nyam et al (2018) | Eric Nyam et al (2018). ‘The Risk of Traumatic Brain Injury Occurring Among Patients with Parkinson Disease: A 14-Year Population-Based Study.’ World Neurosurg; 113:e328-e335. |
| 1.4 van der Marck et al (2014) | van der Marck et al (2014) ‘Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease’ Parkinsonism Related Disorders; 20:360–9 |
2 Understanding osteoporosis in Parkinson’s
Having completed section 1 you should now have:
an overview of the epidemiology of falls in Parkinson’s
an understanding of why people with Parkinson’s fall
an awareness of the potential sequelae of falls
In section 2, the objectives are to gain:
an overview of bone metabolism and structure
an understanding how these are altered in osteoporosis
knowledge of the particular contributors to osteoporosis in Parkinson’s
People with Parkinson’s are at much higher risk of breaking a bone. This increased risk arises partly from the tendency to fall, addressed in the first section. But it also occurs because more people with Parkinson’s have reduced bone strength. To understand why this is, we will look at the structure and metabolism of healthy bone.
2.1 Bone metabolism and structure
Healthy bones provide:
- a strong skeletal structure but to allow efficient movement, weight is minimised
- a site for haematopoiesis (the production of blood cells by the bone marrow)
- a reservoir of calcium
Bone is not a single structure, but has 2 subtypes
Cortical bone
Cortical bone is dense, made of smooth layers of bone tissue and nourished by blood vessels.
Trabecular bone
Trabecular bone consists of a honeycomb-like network of interconnecting bone tissue, interspersed with spaces filled with bone marrow.
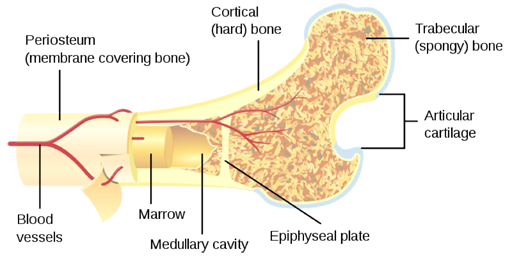
2.1.1 Bone anatomy
About 80% of the body’s bone is cortical, with a long bone like the femur arranged as a cylinder of cortical bone around a core of trabecular bone and marrow. [image as previous]
However some bones, like the spinal vertebrae, constitute predominantly trabecular bone. The strength of trabecular bone depends on the connectivity of the trabeculae.
Bone tissue is a matrix of collagen protein, with minerals, especially calcium, which make the matrix rigid. There are bone cells embedded within this matrix. They make a unit of cells (the basic multicellular unit = BMU) which creates controls and repairs the matrix.
There are 3 cell types in this cellular network of bone:
Osteoblasts: these create new bone by secreting matrix.
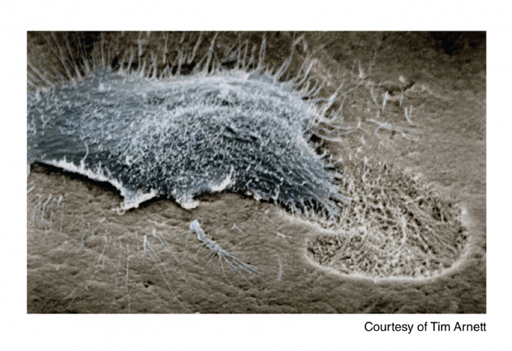
Osteoclasts: these resorb old or damaged bone, by dissolving it.
Osteocytes: these sense bone loading and orchestrate the activities of the other cells
2.1.2 Bone turnover
Both osteoblasts and osteoclasts are continually active in a process called remodelling. Old or damaged bone is removed by osteoclasts (resorption) and replaced by osteoblasts (bone formation). The balance or ‘coupling’ of this activity is crucial to bone strength. Where resorption exceeds formation, bone strength is reduced and osteoporosis may occur.
Some key factors influence this balance of remodelling:
- Hormones
- Parathyroid hormone (PTH)
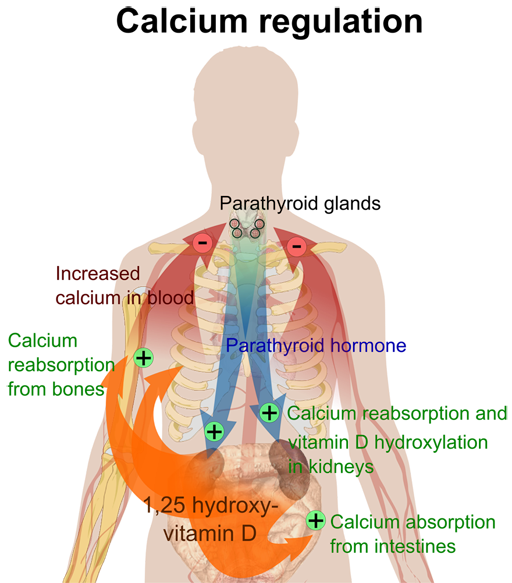
PTH is responsible for the calcium balance of the body and depending on secretion patterns, PTH will either promote bone formation or bone resorption. PTH is elevated when vitamin D or calcium are low in order to draw calcium from the bones into the blood.
- Sex hormones
Oestrogen and testosterone both promote bone formation.
- Thyroid hormone
Thyroid over-activity reduces bone and muscle strength.
- Parathyroid hormone (PTH)
- Mechanical loading
Osteocytes respond to mechanical stress on bone to promote bone formation.
- Cytokines (cell signalling molecules)
RANK-Ligand and other substances secreted by osteoblasts and osteocytes control osteoclast activity.
Please complete the following quiz. This is formative. It is really helpful in consolidating your learning, but there is no pass mark.
So far we have looked at the structure and function of healthy bone. But if the balance of remodelling is disturbed, bone may lose strength and become osteoporotic.
2.2 What is osteoporosis?
There are two, overlapping meanings for this term.
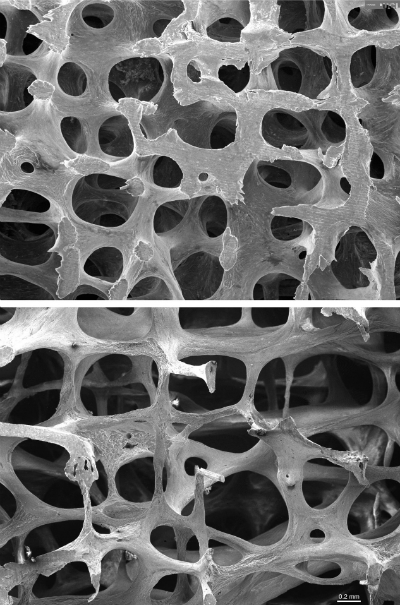
Osteoporosis means ‘porous bones’ and is used to refer to the condition of reduced bone strength in adulthood which makes bones more fragile and easily broken.
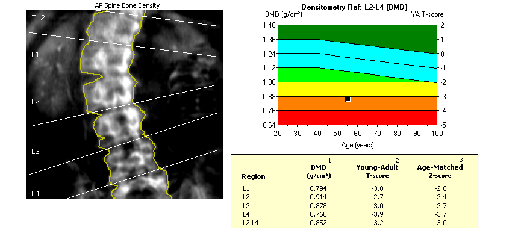
It is also used to refer to a condition of low measured bone mineral density (BMD), far below the normal range of a young woman’s BMD on a DXA scan.
2.2.1 Quantifying osteoporosis
When bone mineral density (on a DXA scan) is more than 2.5 standard deviations below the mean peak bone mass for a young woman, the individual is said to have osteoporosis.
The T-score is the name for the number of standard deviations a person’s BMD is from the young adult mean. So a T-score of -2.5 is the cut-off for defining osteoporosis by BMD.
However, bone strength is a spectrum and many people without a diagnosis of ‘osteoporosis’ by DXA scan have reduced bone strength. Where DXA results are between 1 and 2.5 SD below the young adult mean, this is called ‘osteopenia’. Because osteopenia is more common, and is associated with reduced bone strength, most people who fracture have osteopenia rather than osteoporosis.
2.2.2 Osteoporosis and fragility fractures
Osteoporosis is associated with increased fracture risk, because the reduction in bone strength makes the bones less able to withstand increased load.
When a bone fractures because of minor trauma, such as a fall from standing height or lower (or even simply from a twisting or bending movement or from coughing) it is known as a fragility fracture.
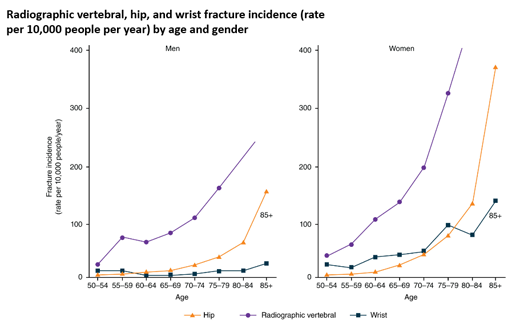
The most common fragility fracture sites are the hip, spine and wrist, although the humerus and pelvis are also frequently affected and any bone can fracture because of osteoporosis. In Parkinson’s, hip fractures are more common, probably because of falling without the protection of outstretched hands.
Any fragility fracture is associated with an increased risk of future fracture. This is true even for asymptomatic vertebral fractures found incidentally (for instance, on a CT scan of the chest).
People with Parkinson’s are more likely to have osteoporosis than others of their age and sex. To understand why, we need to look at the causes of osteoporosis in the general population.
2.2.3 What causes osteoporosis?
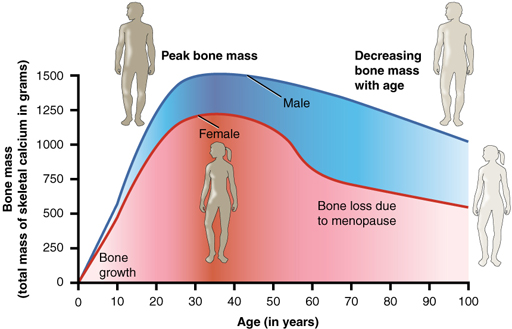
Many factors contribute to the development of osteoporosis. Loss of bone mass starts to occur after the age of 30. Peak bone mass, which is reached in young adulthood, is set by three main factors:
- genetics (which accounts for 70-80% of the variation in peak bone mass)
- diet and lifestyle
- exercise levels
Load bearing exercise also encourages the osteoblasts to form more bone tissue.
The speed of bone loss is influenced by diet, disease and absorption of vitamin D (discussed in 4.2.2). An adequate diet supports the maintenance of a healthy bone matrix and stops the body needing to draw on the calcium reserves in the skeleton. Lifestyle factors including both alcohol and tobacco use lead to lower bone mineral density. Hormones also play a role in this. For example, in women, after the menopause, there is a period of accelerated bone mineral density loss due to the reduction in oestrogen levels. Anti-hormone cancer treatments, such as for prostate or breast cancer, suppress sex hormones and discourage bone formation. Different illnesses speed up bone loss. Many medications can reduce bone density, perhaps, most importantly, steroids and anti-epileptics. Lastly, even without measured low bone mass or density, conditions that cause high bone turnover reduce the structural integrity of bone – particularly of trabecular bone.
2.3 Who develops osteoporosis?
Because peak bone mass in women is lower than in men, and women lose bone mass faster with ageing because of the menopause, osteoporosis is more common in women than men overall. However, people of either sex with some health conditions, including Parkinson’s, are more likely to have osteoporosis.
2.3.1 Risk factors for developing osteoporosis
The majority of cases of osteoporosis in women relate to relatively low peak bone mass achieved in youth. This is usually genetically determined along with normal menopause and normal ageing, as well as a sedentary lifestyle or a less healthy diet. However, other conditions and factors may be important drivers of bone loss, which can then be called secondary osteoporosis. Where there are other important medical factors, patients may benefit from further investigation, either by their GP or a specialist such as a geriatrician or a rheumatologist. In the general population of men with osteoporosis, if investigating for secondary causes, you are more likely to find one than not. Examples of such causes are listed in the table below.
| Examples of secondary causes of osteoporosis: |
| type 1 diabetes mellitus |
| untreated longstanding thyrotoxicosis |
| hypogonadism or premature menopause (below age 45) |
| chronic malnutrition |
| malabsorption |
| liver disease |
| glucocorticoid use |
| alcohol excess |
2.4 Bone health in Parkinson’s
This section has so far addressed the nature of bone health in the general population. What are the specific issues of bone health in Parkinson’s?
Section 1 presented an overview of the risk of falls and consequent fractures in Parkinson’s, particularly hip fracture. A recent systematic review showed that people with Parkinson’s are over twice as likely to have osteoporosis compared to controls (Torsney et al, 2014). Despite this, women with Parkinson’s perceive themselves as ‘as likely or less likely’ to fracture a bone compared with their peers (Gregson et al, 2014). All clinicians have a responsibility to ensure that bone health is addressed in Parkinson’s clinics.
Several factors contribute to reduced BMD and bone strength in Parkinson’s (van den Bos et al, 2013). These include reduction in muscle strength, mobility, load bearing exercise and sunlight exposure. Less vitamin D synthesis occurs as a result. There is also a tendency towards low body mass index and even malnutrition. This is because medications can impact on nutrition, and levodopa has effects on homocysteine handling and collagen cross-linking in bone. Vitamin D is very important to bone health, as it promotes absorption of calcium from the diet and suppression of PTH, tipping the balance in favour of bone formation. 80% of vitamin D comes from the exposure of sunlight on the skin – while about 20% is taken in the diet. Deficiency is common in the general population, especially where sunlight exposure is low. Low vitamin D is associated with poor bone health, as well as several other conditions. People with Parkinson’s have been shown to have low levels of vitamin D compared to the general population (Knekt et al, 2010) and it is good practice to offer vitamin D supplementation to all people with Parkinson’s. Testing vitamin D levels is not required. However, doing this may help encourage some patients to adhere to their prescribed course of vitamin D.
2.5 Activity
Watch this short animation which shows how multiple factors interact to influence bone mineral density and bone strength.
2.5.1 Reflective exercise
Mr Smith is a 74 year old man diagnosed with idiopathic Parkinson’s who has been seen in clinic for the last 8 years. He is taking sinemet 125mg four times a day. He was diagnosed with prostate cancer last year and is now on anti-androgen hormone treatment. He had polymyalgia rheumatica 3 years ago and had steroid treatment for 18 months. He has some back pain. He drinks 3 units of red wine daily. He suffers with constipation which is easily managed with aperients. He is hypothyroid on monitored replacement therapy. He takes bendroflumethiazide for hypertension, zopiclone to aid sleep, as well as aspirin 75mg for secondary cardiovascular disease prevention. He comes to clinic and it is noted that he is shuffling more than he used to and now uses a stick to walk. Coming through the door he gets ‘stuck’ and freezes. There is no ‘wearing off’ phenomenon. On examination he is wearing bifocal glasses, but his eye movements are normal. He has Hoehn and Yahr stage 3 Parkinson’s and has a moderately stooped posture. He has moderate bradykinesia and rigidity of his upper and lower limbs, more evident on the left than the right, and no red flags to suggest an alternative diagnosis to idiopathic Parkinson’s. He weighs 57kg and is 1.59m tall. He reports his main problem as feeling ‘really unsteady’ and as a result he is less confident leaving the house to do his weekly shop. He has not fallen.
Think about what you have learned in Section 2 regarding factors which make someone with Parkinson’s more likely to have reduced bone health. Remember that bone health can be affected both by Parkinson’s factors and other more general factors. There are several things mentioned in the text which may increase Mr Smith’s chance of having reduced bone strength. In your reflective log, identify as many as you can, and make suggestions for reducing their impact. Write down anything else you would like to check.
Discussion
You probably spotted that he has a number of factors including:
- Parkinson’s itself
- levodopa medication
- hormone deprivation therapy
- alcohol intake
- previous steroid treatment
- reduced mobility and therefore likely lower sunlight exposure
NB an overactive thyroid condition (hyperthyroidism) worsens bone health, but underactivity (hyporthyroidism) does not (as long as it is not over-replaced).
You might also want to check if he has a family history of osteoporosis or hip fracture, and whether he has sustained a fracture himself in the past. You could also check that he does not smoke. You might ask whether he was prescribed any bone treatment when he had his steroid therapy, and whether he is taking any over the counter calcium or vitamin D.
2.6 Summary
In the general population, many factors contribute to reduced bone strength and osteoporosis. Parkinson’s can accentuate many of these risk factors and contribute to poor bone health. Ultimately, more fragile bones put people at higher risk of fracture, and the next section will address how we can assess this risk.
2.7 Useful links
2.8 References
| 2.4 Torsney et al (2014) | Torsney KM et al (2014) ‘Bone health in Parkinson’s disease: a systematic review and meta-analysis’ Journal of Neurology, Neurosurgery and Psychiatry; 85:1159–1166 |
| 2.4 Gregson et al (2014) | Gregson CL et al (2014) ‘Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study’ Osteoporosis International; 25:85–95 |
| 2.4 van den Bos et al (2013) | van den Bos F et al (2013) ‘Parkinson’s disease and osteoporosis’ Age and Ageing; 42:156–62 |
| 2.4 Knekt P et al (2010) | Knekt P et al (2010) ‘Serum vitamin D and the risk of Parkinson Disease’ Archives of Neurology; 67:755–761 |
3 Fracture risk assessment
In the last section we reviewed:
- an overview of bone metabolism and structure
- an understanding how these are altered in osteoporosis
- a knowledge of the particular contributors to osteoporosis in Parkinson’s
The objectives for this section are:
- to introduce FRAX and QFracture – the two main risk assessment tools for bone health in Parkinson’s
- to understand the merits of each assessment tool, and the link to National Osteoporosis Guideline Group (NOGG) treatment recommendations
- to introduce a treatment algorithm to guide treatment decisions
3.1 Identifying those at higher risk of fracture
Bone strength and BMD are important predictors of fracture risk. Nevertheless, more fractures occur in people with non-osteoporotic bone (as defined by BMD) than in those with an osteoporosis diagnosis.
Even osteoporotic bone will only break under a degree of trauma and so fall-related factors also contribute strongly to fracture risk.
3.2 Fracture Risk Assessment:
This understanding has led to an increased focus on fracture risk assessment, rather than simply an osteoporosis diagnosis. Accurate prediction of fracture risk allows for appropriate prevention strategies to be tried. This will help to reduce the occurrence of fractures
Fracture risk (expressed as a percentage risk over a period of time) is usually presented as two figures: firstly, a risk or probability of major osteoporotic fractures (MOF), which comprises hip, spine, wrist or humeral fractures, over the defined time frame. Secondly, a risk or probability of hip fracture, over the defined time frame.
Whilst a range of fracture risk assessment tools have been developed, there are just two which are endorsed by the National Institute for Health and Care Excellence (NICE 2017), namely FRAX and Qfracture.
3.2.1 FRAX
The FRAX tool calculates 10-year probabilities for hip and MOF. It can be used for individuals aged between 40 and 90 years. It uses 11 variables:
- age
- sex
- weight
- height
- previous adult fracture
- parental hip fracture
- current smoking
- glucocorticoid (steroid) use
- rheumatoid arthritis
- 3 or more units of alcohol intake daily
- conditions linked with secondary osteoporosis
It uses these variables to calculate the probability of fracture. However, it does not include Parkinson’s or falls as variables. Measured BMD (from DXA scan results) can be included if available but is not essential.
The fracture risk results (displayed as a percentage chance of fracture in the next 10 years) then links directly to guidance from the National Osteoporosis Guidelines Group and displays patient risk on a graph (see later).
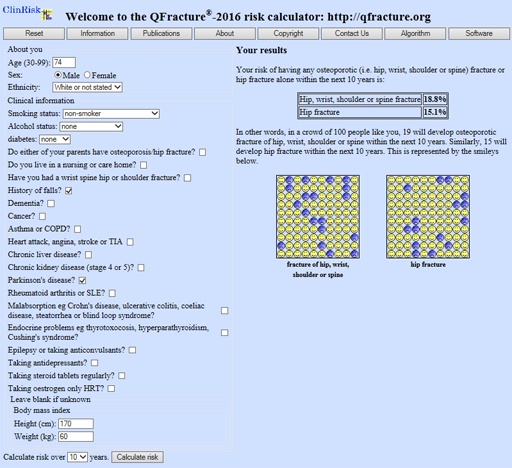
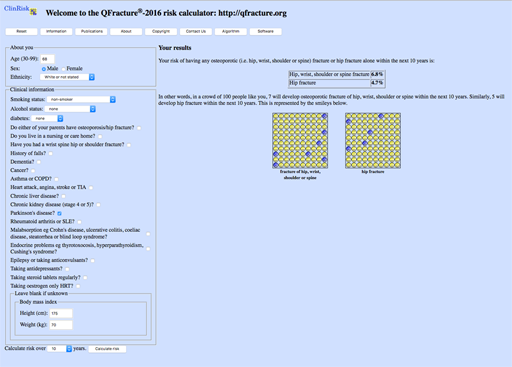
3.2.2 QFracture
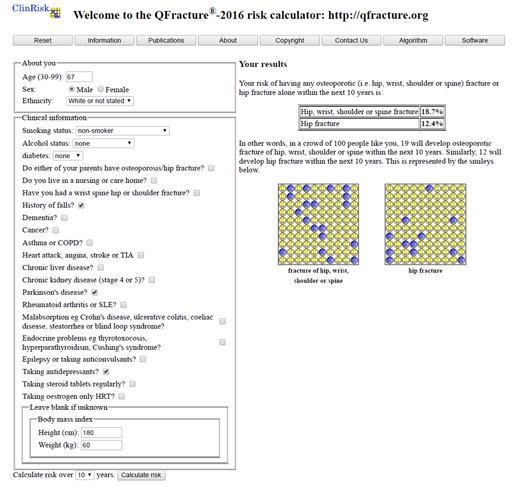
The Qfracture tool calculates risks for hip fracture and MOF. The calculation can be performed for risk over 10 years (as with FRAX) or adjusted to between 1 and 10 years. In older patients or those with a more limited life expectancy, this may aid decision making. Qfracture has the advantage that it is applicable from ages 30 to 99 years. It includes 25 variables; from falls and Parkinson’s, to many other conditions associated with higher fracture risk. It cannot include BMD, even if the patient has had a DXA scan. However, in UK clinical practice many older patients will not have had a DXA. Similarly, it does not link to any guidance and the results must be interpreted by the clinician. It likely overestimates the risk of major osteoporotic fracture.
3.2.3 Activity
Use these example patients to generate risk figures using each tool. Go to the sites (links below) and use the clinical information given to fill in the boxes or answer the questions. If information is missing, as it sometimes is in a clinical setting, then assume a negative answer. Your results should look like this:
| Result: | FRAX MOF: | X% | HIP: X% |
| Qfracture MOF: | X% | HIP: X% |
FRAX Fracture risk assessment tool
QFracture 2016 risk calculator
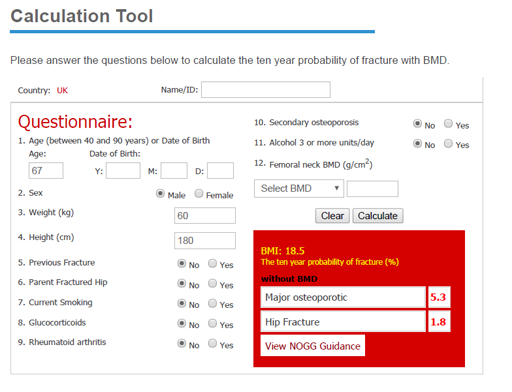
Mr A is 67. He has had Parkinson’s for 9 years, he weighs 60kg, and he is 1m 80. He is a non-smoker and rarely drinks alcohol. He fell twice last year but has never fractured a bone. He has normal cognition but suffers with depression and takes citalopram. He doesn’t think his parents had any fractures.
Mrs B is 74, she was diagnosed with Parkinson’s last year. Her menopause occurred at 42 when she had a hysterectomy. She hasn’t fallen recently. She doesn’t smoke, but drinks about 2−3 bottles of wine per week. She weighs 70kg and is 1m 65 tall. She lives with her husband. She broke her wrist in a fall in her 50s. She thinks that neither parent has broken their hip or had osteoporosis.
If you click the link to NOGG guidance from FRAX, you will see that the graph shows thresholds for intervention, suggesting either lifestyle advice, further investigation with BMD measurement by DXA (if not already done) or treatment. These interventions will be considered in the next section
Activity ii
Redo the examples above using FRAX, and now click the link to NOGG.
Acting on the results.
Once we have assessed fracture risk using these tools, we need to decide what this means for treatment.
The FRAX results offer a link to the NOGG page.
This plots the derived MOF figure on a graph (not the hip figure, though this can be done as ‘manual data entry’ NOGG manual data entry where BMD is known).
What management is suggested for Mr A?
Answer
Based on his MOF score of 5.3%, he is only recommended lifestyle advice.
What management is suggested for Mrs B?
Answer
She is recommended for a DXA, based on her MOF score of 19%.
3.3 Adapting these tools to Parkinson’s
As FRAX does not recognise the particular risks to bone health in Parkinson’s, there are some strategies which can be used to adapt the FRAX tool for the condition. Considerations have been highlighted below in KEY POINT boxes for reference in the next section, where a protocol for managing fracture risk is presented.
3.4 An approach to fracture risk assessment in Parkinson’s
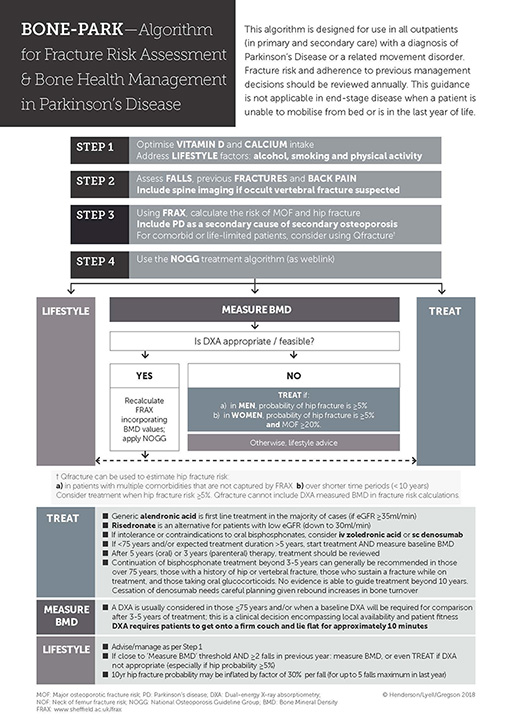
In light of the particular fracture risks in Parkinson’s, Lyell et al (2015), suggested an algorithm for assessing and responding to Parkinson’s fracture risk. In 2017 the NOGG thresholds were updated and, accordingly, a revised algorithm was proposed (Henderson et al, 2019). This protocol is shown in the diagram below and is applicable to people with Parkinson’s, who are seen in primary or secondary care settings, and who are not in the very final stages of illness. It proposes a 4-step approach to ascertaining fracture risk that will then serve to inform treatment options.
Accessible Powerpoint version of image
Step 1 is to address lifestyle factors such as smoking, alcohol use and physical activity. More detail is provided in Section 4.1. Vitamin D and calcium intake should be optimised and this is described in Section 4.2.
Step 2 is to assess the number of previous falls. Some patients may have vertebral fractures but be unaware of them. If they complain of back pain, or have a flexed posture which is not correctable, consider requesting X-rays of the spine. If a vertebral fracture is identified, this can be incorporated into risk assessment, where it will increase future fracture risk scores.
Step 3 FRAX should be used to calculate fracture risk. There are some particular aspects of using FRAX in PD that should be considered.
FRAX is, broadly speaking, the best tool to use to calculate fracture risk in PD. However, QFracture should be considered in patients with a limited life expectancy as risk can be calculated over a shorter period of time (<10 years). In addition, Qfracture facilitates the inclusion of multiple comorbidities and the number of falls an individual has experienced.
As falls are a major fracture risk and common in Parkinson’s, FRAX probabilities could be inflated in the presence of recorded falls (Masud et al, 2011).
KEY POINT
Each fall, up to a maximum of 5, in the last year, can be used to inflate the calculated probability by 30%.
For example, if the FRAX calculated MOF probability was 15% over 10 years, but the patient had fallen twice in the last year, 30% of this risk (4.5%) would need to be added on for each fall. This would make the total risk 15 + 4.5 + 4.5 = 24%.
The clinician can then interpret the inflated probability against NOGG thresholds. Whilst this may be too cumbersome to use routinely, it underlines the point that FRAX underestimates risk in fallers and it is reasonable to allow for this added risk when making decisions.
Secondly, Parkinson’s is associated with poor bone health with a low BMD. Parkinson’s is currently not included in the list of secondary osteoporosis conditions in FRAX.
However, the wording ‘enter yes if the patient has a disorder strongly associated with osteoporosis’ allows for Parkinson’s to be included by proxy. When using the FRAX tool in Parkinson’s the question 10, ‘secondary osteoporosis’ box should be ticked. New NOGG guidance acknowledges the increased risk in Parkinson’s too.
KEY POINT
Selecting ‘YES’ to the FRAX question 10 ‘Secondary osteoporosis’, allows Parkinson’s to be considered as a condition that is strongly associated with osteoporosis.
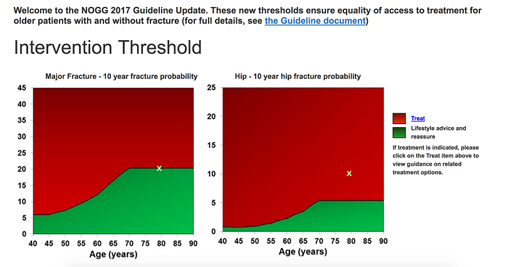
Step 4. Using the NOGG treatment algorithm to guide your management. This graph is found if you click the link after the FRAX results.
This graph relates to MOF risk, rather than hip fracture risk. This can disadvantage those with Parkinson’s in whom hip fracture risk is disproportionately high. The body of the NOGG guidance supports the use of a threshold of 5.4% for hip fracture risk over the age of 70 (there are lower, age specific thresholds for younger patients – see image ‘Hip fracture thresholds NOGG’). It also supports the use of a threshold of 20.3% for MOF risk.
KEY POINT
It is therefore reasonable to treat and/or measure BMD in those with a probability of hip fracture above 5.4%, even if the probability of MOF is not above the intervention threshold on the NOGG guidance graph.
For patients under 70, you should obtain a DXA and recalculate FRAX including the measured BMD. Your patient’s results can be plotted on this graph using the NOGG Manual Data Entry form and entering the MOF and hip fracture probabilities. This will indicate whether your patient should have lifestyle advice only, or be recommended for treatment. There is also a link to straightforward treatment options. In the example image here, you can see that the patient is recommended for treatment based on the hip fracture probability, although the Major Fracture probability is not above the threshold.
If these thresholds are not reached, but MOF probability is over 11%, it is appropriate to obtain BMD measurement and recalculate FRAX. For younger people, these thresholds are lower and increase with age.
3.4.1 Activity
Below are four example patients. Use these to generate risk figures using each tool. Not all patients would need a Qfracture score (the algorithm suggests it only for co-morbid or life-limited patients) but it is useful practice to perform it, and think about whether you would do this in practice. Go to the sites (links below) and use the clinical information given to fill in the boxes or answer the questions. If information is missing, as it sometimes is in a clinical setting, then assume a negative answer.
FRAX Fracture risk assessment tool
QFracture 2016 risk calculator
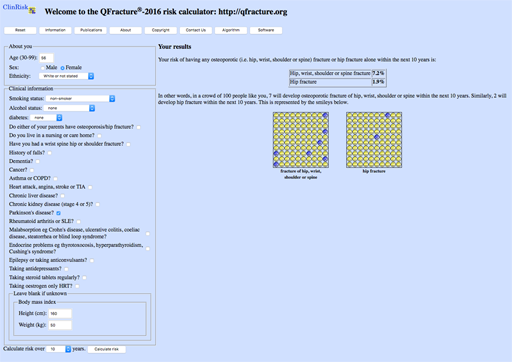
Mr C
Mr C is 68, independent at home, and has had Parkinson’s for a number of years. He is on herbal treatment for depression and has chronic kidney disease stage 3a and hypertension. He has had no previous falls. He weighs 70kg and he is 1m 75 tall.
Answer
| Result: | FRAX MOF: | 7.6% | HIP: 2.3% |
| Qfracture MOF: | 6.8% | HIP: 4.7% |
You can see that Mr C’s risk is below the ‘rule of thumb’ thresholds mentioned above. Using the algorithm suggested, you can follow that NOGG guidance would be simply to give lifestyle advice (more on this in the next section). Qfracture gives a pictorial illustration of his risk but as he is unlikely to have a life expectancy below 10 years there is no particular reason to use Qfracture. His risk should be reviewed yearly as it will change with age and other factors.
Mr D
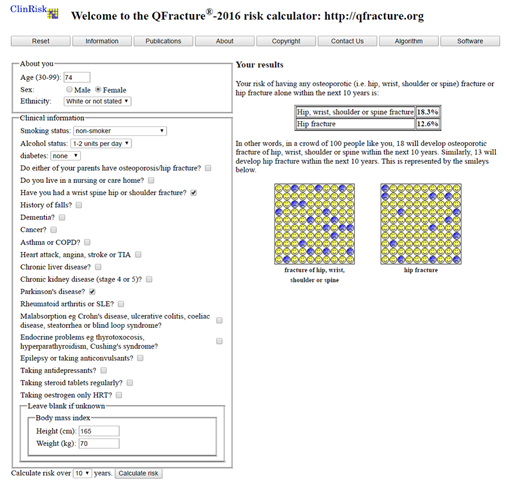
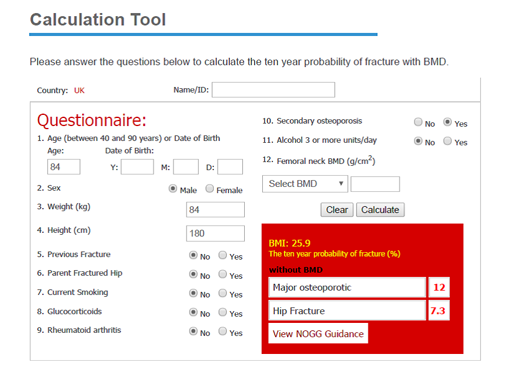
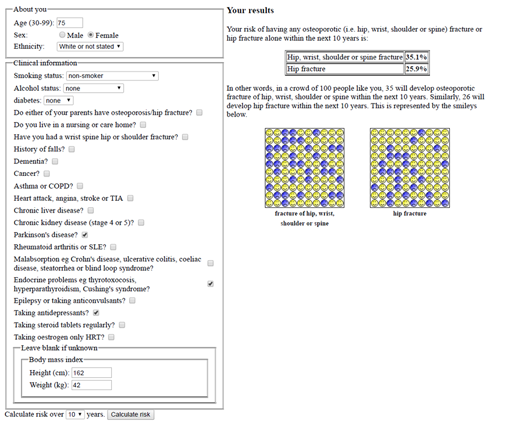
Mr D is 84 years old with Parkinson’s and dementia. He is a residential care home resident and has had 4 falls in the last year. His height is 180cm and he weighs 84kg. He has had a previous transient ischaemic attack (TIA).
Answer
| Result: | FRAX MOF: | 12% | HIP: 7.3% |
| Qfracture MOF: | 36.1% | HIP: 34.5% |
You can see that Mr D’s FRAX MOF risk is below the ‘rule of thumb’ threshold mentioned above, but his hip fracture risk is above 5%. Qfracture gives a very high estimate for his risk (probably an overestimate but clearly raising the question over treatment to reduce fracture risk). Using the algorithm suggested, you can follow that NOGG guidance would be to obtain a DXA. In someone frail and living in a care home, this may not be appropriate or feasible, but the algorithm makes it clear that it would be reasonable to treat according to his hip fracture risk without further investigation.
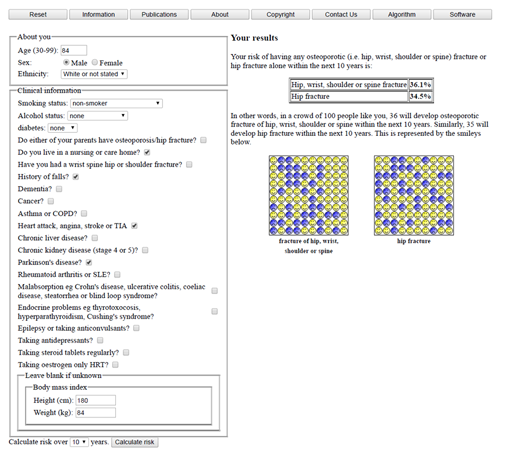
Mrs E
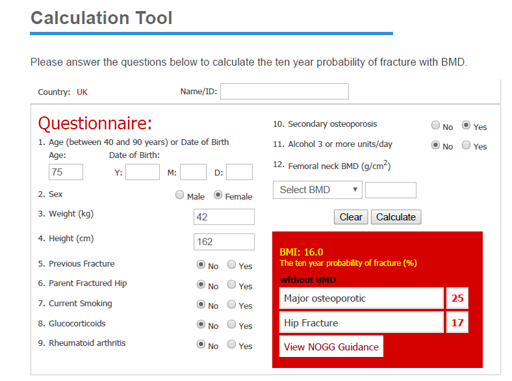
Mrs E, who is 75, has had Parkinson’s since her 60s. She has a history of thyrotoxicosis. She has suffered with depression and takes an SSRI. She weighs 42kg and she is 162cm tall. She has had no falls. She is a non-smoker and drinks no alcohol. She has not had any previous fractures.
Answer
| Result: | FRAX MOF: | 25% | HIP: 17% |
| Qfracture MOF: | 35.1% | HIP: 25.9% |
You can see that Mrs E’s risk is well above the ‘rule of thumb’ thresholds mentioned above. Qfracture gives a pictorial illustration of her risk. Using the algorithm suggested, you can follow that NOGG guidance would be simply to treat, without the need for a DXA. However, presuming she has a life expectancy of more than 5 years, a DXA would be ideal to monitor treatment response and guide decision making after 5 years of treatment. There is no need to await the DXA results before starting treatment.
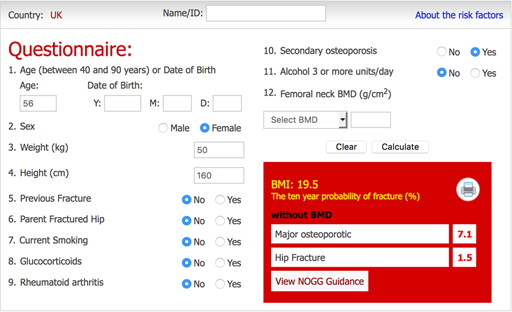
Mrs F
Mrs F is 56, with Parkinson’s but no other health issues. She had an early menopause at 44 but was on HRT until the age of 50. She weighs 50kg and she is 160cm tall.
Answer
| Result: | FRAX MOF: | 7.1% | HIP: 1.5% |
| Qfracture MOF: | 7.2% | HIP: 1.9% |
You can see that Mrs F’s risk is below the ‘rule of thumb’ thresholds mentioned above. Using the algorithm suggested, you can follow that NOGG guidance would be simply to give lifestyle advice (more on this in the next section). Qfracture gives a pictorial illustration of her risk but does not need to be performed unless this is helpful to her. Her risk should be reviewed yearly as it will change with age and other factors.
3.5 Summary
In this section, you have learnt about the importance of overall fracture risk assessment, the tools in common use to assess risk and an algorithm to follow to promote bone health in Parkinson’s. In the next section, we will examine the treatment options available for bone health.
3.7 References
| 3.2 NICE (2017) | National Institute for Health and Care Excellence (2017) Parkinson's disease in adults Available at: https://www.nice.org.uk/guidance/NG71 [accessed December 2020]
|
3.3 Masud et al (2011) | Masud T et al (2011) ‘FRAX(®) Position Development Conference members, official positions for FRAX® clinical regarding falls and frailty: can falls and frailty be used in FRAX®?’ Journal of Clinical Densitometry; 14:194–204 |
3.4 Lyell et al (2015) | Lyell V et al (2015) ‘Assessment and Management of Fracture Risk in Patients with Parkinson’s Disease’ Age and Ageing; 44:34–41 |
3.4 Henderson et al (2019) | Henderson et al (2019) ‘Management of fracture risk in Parkinson's: A revised algorithm and focused review of treatments’, Parkinsonism & Related Disorders, 64, 181 - 187 |
4 Treatment options for osteoporosis in Parkinson’s
In the previous section, you learnt about assessing fracture risk in Parkinson’s. This section looks at treatment options for osteoporosis and for reducing fracture risk, with particular considerations for Parkinson’s.
In section 4, you will gain:
- knowledge of the components of appropriate bone health lifestyle advice and vitamin D management
- familiarity with the classes of drugs that are used to treat osteoporosis and reduce fracture risk as well as the mechanisms of drug action
- awareness of the suitability of bone-specific treatment options in Parkinson’s, including the interaction of side effects and non-motor symptoms
As you have seen, osteoporosis and increased fracture risk have several contributing factors and many are amenable to lifestyle advice. This is as true in Parkinson’s as it is in the general population, and Parkinson’s clinicians are well placed to reinforce this advice. There are good resources for patients and clinicians from the Royal Osteoporosis Society (ROS). Everyone at risk of fracture should be encouraged to maximise their bone health by paying attention to the following factors.
4.1 Lifestyle measures
There are a number of lifestyle measures that should be considered.
4.1.1 Exercise
There are many reasons to promote exercise in Parkinson’s, and bone health is one important factor. The physical impact of weight bearing exercise (like walking, running, dancing, or anything which puts load through the bones) causes bone cells to signal for increased osteoblast activity, which increases bone density and strength.
4.1.2 Falls
By building muscle strength and improving balance, exercise can also reduce both falls risk and the chance of fracturing. People with Parkinson’s should be regularly reviewed to consider whether a falls assessment and/or strength balance programme should be offered (see section 1).
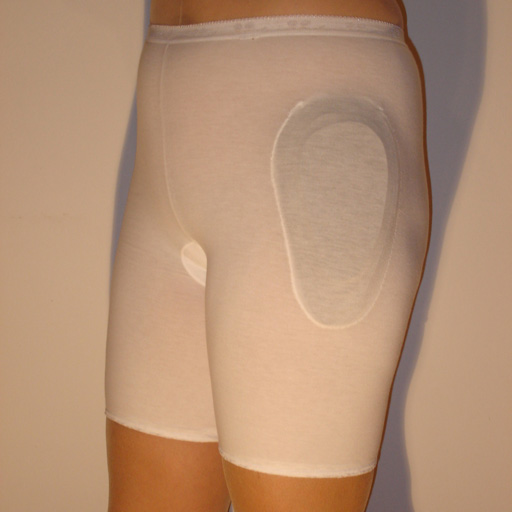
4.1.3 Hip protectors
For several years, padded or reinforced underwear or outerwear called hip protectors have been available. There is little evidence for their routine use (Gillespie et al, 2010) but in motivated people, hip protectors may be reassuring and could reduce the chance of fracture.
They are available to purchase from mobility shops and online but they are not available on prescription.
4.1.4 Smoking
Smoking reduces the activity of osteoblasts (see section 2) and so lowers bone mineral density and doubles hip fracture risk (Dimai & Chandran 2011). Anyone who smokes should be offered advice and support to quit.
4.1.5 Alcohol
Excessive alcohol intake raises fracture risk by its dual action on falls risk and on bone health. The government’s current advice is to keep intake below 14 units per week and to spread any drinking over the week.
4.2 Diet, vitamin D and calcium
It is also important to consider the patient’s diet, vitamin D and calcium intake.
4.2.1 Diet
Healthy eating is good for everyone, and a balanced nutritional intake promotes a healthy weight too. Parkinson’s can affect nutritional status for several reasons and people with the condition may be underweight which is itself a risk factor for fracture. Signpost patients to sources of information such as Parkinson’s UK Diet and Parkinson’s information and ROS Nutrition for bones and consider referral to a dietician where needed.
4.2.2 Vitamin D
Vitamin D helps regulate calcium handling and plays an essential part in the health of bones, muscles and teeth. For healthy adults in the UK, most vitamin D (~90%) is obtained by the exposure of sunlight to the skin. The sun’s ultraviolet light makes a relatively inactive form of vitamin D (cholecalciferol/vitamin D3) which is converted into an active form of vitamin D principally by the kidneys. The unactived form can also be obtained through diet (but doses are relatively small) or may be supplemented. The ROS leaflet ‘Nutrition for bones’ has more information.
People with reduced sunlight exposure are at risk of vitamin D deficiency, which is common in the UK. Government guidance has recently increased the list of people recommended for supplementation (see ROS website or direct to SACN report).
It is good practice to offer vitamin D supplementation, without the need for testing levels. However, testing may remain appropriate in some people, for example:
- where a patient is reluctant to take supplements, unless deficiency is demonstrated
- where supplements are prescribed but there are concerns about compliance
- where more potent anti-resorptive drug treatments such as Denosumab or i.v. Zoledronic Acid are planned. In these cases, vitamin D must be checked and if necessary replaced before starting treatment
Does the patient require vitamin D maintenance or replacement therapy?
Vitamin D insufficiency is common in Parkinson’s and most patients are not on adequate replacement (Luthra et al, 2018). For most people with Parkinson’s, vitamin D supplementation, to maintain vitamin D levels, will take the form of oral medication containing 800iu of cholecalciferol (vitamin D3) daily. This may or may not be in combination with calcium. Many different forms of such medication exist (soluble/chewable/tablets) and it is usually possible to find one which suits the patient.
Such maintenance treatment may slowly correct low vitamin D levels, although usually higher dose replacement is needed to correct vitamin D deficiency. Where vitamin D is known to be low, and particularly if a potent ‘anti-resorptive’ medication is to be prescribed (see below) then replacement is required more rapidly. It is sensible to ask GPs to check calcium levels in the blood one month after starting treatment. This is because in rare cases an underlying condition called primary hyperparathyroidism can be revealed by the correction of low vitamin D levels. For this same reason, don’t replace vitamin D if the calcium level is high – specialist advice should be sought in this rare scenario.
An example of an appropriate vitamin D replacement schedule is shown below:
| Treatment | Example regime | |
|---|---|---|
Deficiency <30 nmol/l | 300,000 IU then maintenance | 50,000 IU weekly for 6 weeks then 800-1,600 IU/day |
Insufficiency <50 nmol/l | 150,000 IU then maintenance | 25,000IU weekly for 6 weeks then 800-1,600 IU/day |
Sufficiency (>50nmol/l) | maintenance | 800-1,600 IU/day |
These higher dose supplements are currently licensed as Fultium but other versions are available depending on local prescribing guidance. Calcium levels should be checked one month after starting these replacement schedules. Retesting of vitamin D levels is not needed unless there are concerns about compliance.
4.2.3 Special circumstances
As explained above, vitamin D3 is activated principally by the kidney. In kidney disease this activation may fail. In these circumstances, patients may need supplementation with an activated form of vitamin D, such as alfacalcidol. This will require closer monitoring, which will normally be under the guidance of a specialist physician. A specialist should be consulted about giving vitamin D to patients with eGFR<30ml/min if there is a history of:
- renal stones
- hyper/hypocalcaemia
- hyperparathyroidism
- sarcoidosis
- lymphoma
- metastatic cancer
- active TB
- skeletal deformity
- chronic liver disease
- malabsorption (e.g. coeliac disease)
4.2.4 Calcium
Calcium provides rigid cross-links within the bone tissue and most of the body’s store of calcium is found within the skeleton. Calcium is also vital to many cellular functions and if necessary the body will draw on the bone stores of calcium to keep blood levels in the normal range. So a diet low in calcium can compromise bone health.
The ROS leaflet in Section 4.2.2 gives useful advice about ensuring adequate dietary calcium. People can also use a ‘calcium calculator’ online to estimate their personal intake. Where diet is not providing enough calcium, supplementation can be provided, usually in combination with vitamin D3, and such treatments come in many formats (see above).
All the pharmacological treatments for bone health described below were trialled in combination with calcium and vitamin D supplementation. As such, it is usual to co-prescribe these unless there is demonstrably adequate calcium and vitamin D intake.
Reflective activity
Apply the calcium calculator to yourself. Are you getting enough calcium from your diet?
In your reflective log describe what advice would help you to improve your diet for bone health, if needed?
4.3 Drug treatment for osteoporosis: bisphosphonates
Bisphosphonates are the most common drugs that are used to treat osteoporosis and reduce fracture risk. They are anti-resorptives, which work by preventing the action of osteoclasts (as discussed in section 2) and so reduce bone re-modelling, with a shift towards bone formation and increased BMD. They reduce fracture risk substantially, almost halving risk over 3 years.
Bisphosphonates can be administered orally on a weekly basis. The most potent is alendronate, which is licensed for post-menopausal women, men with osteoporosis, and to prevent glucocorticoid (steroid) induced osteoporosis. It has been shown to reduce fractures to the hip, spine and wrist. Risedronate has the same licence and pattern of efficacy though is slightly less potent. There is also a monthly preparation (ibandronate) but there is less evidence in its favour and it is only licensed in women to prevent vertebral fractures.
Bisphosphonates are poorly absorbed, especially if taken with any food. They can also irritate the oesophagus. To avoid these problems patients must be told how to take the drug effectively:
- first thing in the morning, an hour before breakfast or before any other food, drink or tablets
- washed down with a whole glass of water (not orange juice or any other drinks), to ensure the tablet passes through the oesophagus and into the stomach
- remaining upright (sitting or standing is fine, but not bending over or lying down) for 30-60 minutes to avoid the drug refluxing into the oesophagus.
The dosage instructions for oral bisphosphonates can be challenging, especially in Parkinson’s where people may already have swallowing problems or cognitive difficulties. The most common side effect is gastrointestinal upset, especially dyspepsia. If patients get significant symptoms, they should suspend treatment and consult their GP. However, most people tolerate these medicines without side effects, and if they understand the need for such treatment then adherence can be good.
More information is available from the Royal Osteoporosis Society in their patient fact sheet, Alendronate (alendronic acid or Fosamax)
The main contraindications to bisphosphonates are renal disease (avoid if eGFR<35 ml/min for Alendronate and avoid if <30 for risedronate ml/min) and oesophageal pathology. NOGG guidance also recommends dental examination with preventive dentistry prior to treatment with oral or intravenous bisphosphonates and further detail is available in section six of the NOGG full guideline.
Where the oral bisphosphonates are not tolerated (most commonly for swallowing, oesophageal or compliance issues), an intravenous preparation of bisphosphonate (zoledronate) can be used instead. This is now low cost, and even more potent at reducing fracture risk. However, in most areas its administration requires referral, usually to rheumatology services, though it is likely to become more widely available. The dose is repeated once yearly for 3 years. It cannot be used where the eGFR is below 35 ml/min. It is essential that vitamin D deficiency is corrected before administration, to avoid hypocalcaemia. The main side effect of intravenous bisphosphonates, affecting up to 40% of patients, is flu-like symptoms that can last 1-3 days, but which can be managed with simple analgesia such as paracetamol and good hydration.
4.3.1 Rare but serious side effects of bisphosphonates
Bisphosphonates are known to be occasionally associated with a problem with bone and gum healing in the jaw, called osteonecrosis of the jaw (ONJ). This is more often associated with the high doses of bisphosphonates used in cancer treatment, but patients with poor dentition or significant planned dental work should not be prescribed intravenous bisphosphonates.
In rare cases the effective suppression of bone turnover is thought to increase the risk of a particular type of fracture in the thigh, called an atypical femoral fracture. This is less rare with long courses (>7 years) of bisphosphonates. But for those at high general fracture risk the benefits of bisphosphonate treatment far outweigh the risk of this rare possible side effect. For those at lower risk, reassessment of treatment after 5 years is recommended and there is fuller advice on this, and on treatment monitoring, within section 7 of the NOGG full guidance.
4.4 Quiz 3
Please complete the following quiz. This is formative. It is really helpful in consolidating your learning, but there is no pass mark.
4.5 Drug treatment for osteoporosis: Denosumab
Denosumab is another anti-resorptive but as a human monoclonal antibody, has a different mechanism of action from the bisphosphonates. Denosumab was launched in 2010 for the treatment of osteoporosis. The cells that make osteoclasts have receptors on their surface called RANK (receptor activator of nuclear factor-kappa B), which promotes the development of mature osteoclasts. It is the ligand to this which is inhibited by denosumab, thereby reducing bone loss.
Patients are given 60mg subcutaneously every 6 months. The most common side effects are joint and muscle pain in the arms or legs. There is an increased risk of infections such as cellulitis of hypocalcemia (low blood calcium) and hypersensitivity allergy reactions. There is also, as with bisphosphonates, a very small risk of atypical femoral fractures and osteonecrosis of the jaw (ONJ).
4.6 Drug treatment for osteoporosis: other treatment options
Other treatments are available including selective oestrogen reuptake inhibitors (SERMs) including raloxifene, oestrogens and teriparatide. There are however significant factors that limit their applicability meaning that their use is confined to specialist units.
4.7 Treatment duration and adherence
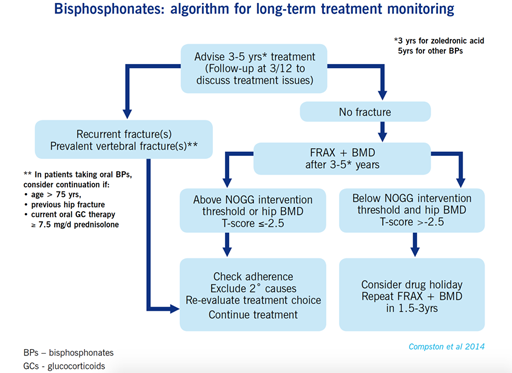
A treatment review should be performed after 3 years of zoledronic acid therapy and 5 years of oral bisphosphonate treatment.
Continuation of bisphosphonate treatment beyond 3-5 years can generally be recommended in individuals age >75 years, those with a history of hip or vertebral fracture, those who sustain a fracture while on treatment and those taking oral glucocorticoids (steroids). A repeat BMD measurement can be helpful in reassessing risk. More detailed advice, and the following algorithm, can be found in section 7 of the NOGG full guidance.
If treatment is discontinued, fracture risk should be reassessed after a new fracture, regardless of when this occurs. If no new fracture occurs, assessment of fracture risk should be performed again after 18 months to 3 years.
The beneficial effects of denosumab wear off very quickly once it is stopped, so specialist advice should be sought when doing this.
People with Parkinson’s can find concordance with their medication schedule challenging. Adding osteoporosis treatment to an often, already, complicated regime, presents a further challenge and burden. Aligning medication timings can help adherence and the use of a combination of pill organisers, reminders and alarms can help concordance with medication schedules (Straka et al 2018). Bisphosphonate compliance is often sub-optimal and it is imperative to review this at follow-up. Non-concordance is often secondary to mild gastrointestinal side effects and intravenous zoledronic acid can be considered in these circumstances.
4.8 Summary
In this section you have learnt the components of appropriate bone health lifestyle advice and vitamin D management. You have become familiar with the classes of drugs that are used to treat osteoporosis and the mechanisms of drug action. You have gained an awareness of the suitability of specific osteoporosis treatment options in Parkinson’s, including the interaction of side effects and non-motor symptoms.
4.9 Useful links
Royal Osteoporosis Society (ROS)
Parkinson’s UK Diet and Parkinson’s booklet
4.10 References
| 4.1 Gillespie et al (2010) | Gillespie W et al (2010) ‘Hip protectors for preventing hip fractures in older people’ Cochrane Database Systemic Review; 3 |
| 4.1 Dimai & Chandran, (2011) | Dimai HP & Chandran M (2011) ‘Official Positions for FRAX® Clinical Regarding Smoking’ Journal of Clinical Densitometry; 14:190–193 |
| 4.2 Luthra et al (2018) | Luthra et al (2018). Characterization of vitamin D supplementation and clinical outcomes in a large cohort of early Parkinson’s disease. J Clin Mov Disord 5, 7 |
| 4.7 Torre et al (2019) | Torre et al (2019) Low persistence with oral bisphosphonate treatment in postmenopausal osteoporosis Acta Reumatol Port; 44:114-125 |
| 4.7 Straka et al (2018) | Straka et al (2018). Clinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic review. Medicine, 97(23). |
5 Summary
Congratulations – now that you have completed section 4 you have reached the end of this course. You have learnt about falls risks, osteoporosis, fracture risk assessment and bone health management options in people with Parkinson’s. You have passed your final assessment and we hope that you will now apply your learning to your clinical practice to the benefit of your patients.
Transcript
You have now completed all the modules of the Parkinson's bone health course. There is a certificate called a statement of participation, which you can print out. We’d really welcome any feedback, which we can incorporate into future iterations of these modules, so please contact us through the Parkinson's UK team.
We hope that the Parkinson's bone course has provided you with a greater understanding of the importance of bone health in Parkinson's and shown you that there are real opportunities to reduce fracture risk in our patients. In order that your patients have the best possible chance of avoiding the painful and distressing consequences of broken bones, particularly broken hips, we now hope that you feel empowered to take these opportunities.
End of course quiz
You may want to review all or particular parts of the course before taking the final test. You need to take the quiz to earn the badge, but it is also important to review the course for your learning.
It may also be helpful to review your reflection log before taking the final test. This will remind you of your thoughts on particular areas of learning and practice.
Now try the end of course quiz
Consolidating your learning
Everything you have learned in this course is important for your practice. How you apply this knowledge to your role will determine how well you can provide support to improve the lives of people affected by Parkinson’s and their families.
You know your own role and how best to use the knowledge gained from the course to make positive practical changes.
Feedback and find out more
The Open University is committed to supporting students from a wide range of backgrounds and circumstances. The UK Parkinson’s Excellence Network brings together health and social care professionals to transform care for people affected by Parkinson’s.
It would be great to receive your feedback about this course. We are keen to know about the parts you found useful and where you feel we can improve. You can share your views through our short survey– thank you in advance for completing it.
The UK Parkinson’s Excellence Network offers resources to support service improvement and engage people affected by Parkinson's, comprehensive information about education and training, and collaboration opportunities. We recommend that you visit the website and sign up for the newsletter to receive regular updates.
Find out more: parkinsons.org.uk/ excellencenetwork
Glossary
- BMD
- bone mineral density, measured by DXA scan. It is usually expressed as a T-score, which indicates how far the patient’s BMD is below (or above) the young woman average.
- DXA
- dual energy absorptiometry scan which measures BMD. It is very low radiation (comparable to background levels) and takes a few minutes. Patients must be able to get on and off a couch.
- Osteoporosis
- meaning ‘porous bones’. It is used to refer to the condition of reduced bone strength in adulthood which makes bones more fragile and easily broken. It is also used to refer to a condition of low measured bone mineral density (BMD), below the normal range of a young woman’s BMD on a DXA scan.
- Osteopenia
- where DXA results are between 1 and 2.5 SD below the young adult mean.
- Secondary osteoporosis
- where specific medical conditions play a significant role in reducing bone mineral density. This is different to primary osteoporosis, which relates mainly to age, genetic predisposition, and diet and lifestyle factors.
- Osteoblast
- these bone cells create new bone by secreting a collagen and mineral matrix.
- Osteoclast
- these bone cells resorb old or damaged bone, by dissolving it.
- Osteocyte
- these bone cells sense bone loading and orchestrate the activities of the other cells.
- Trabecular bone
- this consists of a honeycomb-like network of interconnecting bone tissue, interspersed with spaces filled with bone marrow.
- Cortical bone
- this bone is denser, made of smooth layers of bone tissue and nourished by blood vessels. It forms the outer structure of bones.
- Androgen
- a term for male sex hormone, predominantly testosterone.
- FRAX
- a fracture risk assessment tool recommended by NICE. It links to National Osteoporosis Guidelines Group (NOGG) guidance.
- Qfracture
- a fracture risk assessment tool recommended by NICE. It is not linked to any specific treatment advice.
- NOGG
- National Osteoporosis Guidelines Group – a multidisciplinary group created to provide guidance to users of the FRAX tool. Its processes are accredited by NICE.
- Major Osteoporotic Fracture (MOF)
- this term encompasses hip, spine, wrist or humeral fractures
- Anti-resorptive
- a drug which reduces the action of osteoclasts in the bone, tipping the balance of activity towards formation
- Bisphosphonates
- the commonest osteoporosis treatments, acting on osteoclasts as anti-resorptives. They are available as oral or intravenous preparations.
- Osteonecrosis of the jaw (ONJ)
- a rare side effect of bisphosphonate therapy, seen mainly in high dose anti-cancer use, in which the gum fails to heal over exposed bone.
Acknowledgements
This course was written by Emily Henderson and Veronica Lyell with guidance from Celia Gregson and assistance from Claire Hewitt (Parkinson’s UK), Pete Cannell and Ronald Macintyre (The Open University). This course is part of the Opening Educational Practices in Scotland Project.
Dr Emily Henderson is a Consultant Geriatrician at the Royal United Hospital Bath. She is a specialist in Parkinson’s and related disorders and along with her clinical work, is based at the University of Bristol pursuing research interests in falls, cognition and Parkinson’s.
Dr. Veronica Lyell is a Consultant Physician and Orthogeriatrician in the Royal United Hospital Bath NHS Foundation Trust. She has a particular interest in bone health in Parkinson’s.
Dr Celia Gregson is a Consultant Senior Lecturer in Musculoskeletal Medicine at the University of Bristol and Consultant Physician and Orthogeriatrician at the Royal United Hospital Bath NHS Foundation Trust. Her specialist interests include osteoporosis, fracture risk and muscle and bone health.
Particular thanks and appreciation go to the J. Macdonald Menzies Trust who provided the funding to enable the development of this course.
The following materials in this course are all rights reserved. Please apply to the copyright holder to reuse these materials.
All images in this course, unless otherwise attributed, belong to Parkinson’s UK. Please contact Parkinson’s UK if you wish to reuse any of the images.
All videos in this course belong to Parkinson’s UK. Please contact Parkinson’s UK if you wish to reuse any of the videos.
The cortical bone diagram in section 2.2 is reproduced with permission from International Medical Press. © International Medical Press, 2017. All rights reserved.
The image of example results from the Qfracture risk calculator in section 3.3.3 is reproduced with permission from ClinRisk Ltd. © ClinRisk Ltd, 2017. All rights reserved.
The 2017 bone health algorithm image in section 3.5 is reproduced with permission, courtesy of Veronica Lyell, Royal United Hospital Bath and Celia Gregson, University of Bristol and Royal United Hospital Bath © 2017. All rights reserved.
The comparison of normal and osteoporotic bone architecture image in section 2.2.1 is reproduced with permission, courtesy of Prof Tim Arnett, University College London.
The magnified image of an osteoclast image in section 2.2.1 is reproduced with permission, courtesy of Prof Tim Arnett, University College London.
The anonymised result from DXA scan of a spine in section 2.3.1 is reproduced with permission, courtesy of Jackie Shipley, Royal National Hospital for Rheumatic Diseases.
The demographics of wrist and hip fracture image in section 2.3.2 is reproduced with permission, courtesy of Tjeerd van Staa.
The NOGG MOF and Hip graph in section 3.4 is reproduced with permission, courtesy of Prof E V McCloskey, University of Sheffield.
The age and bone mass image in section 2.3.3 is reproduced courtesy of the Anatomy & Physiology, OpenStax. This work is licensed by Rice University under a Creative Commons Attribution License (by 4.0).
The hip protector image in section 4.2.3 is reproduced with permission, courtesy of Lkshlzr at English WikipediaThis file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. Subject to disclaimers.
Except for third party materials and where otherwise stated in the acknowledgements section, this content is made available under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Licence.
This course is written for a UK audience and has taken care to address terminology, guideline and policy differences. Participants are responsible for recognising country differences and adapting to their own context.
The course may, from time to time, contain links to other (third party) websites. These links are not provided by the authors as a guarantee or recommendation of the services, information, opinion or any other content on such websites or as an indication of any affiliation, sponsorship or endorsement of them. We take no responsibility for clinical decisions made on the basis of this information.
Don’t miss out:
The UK Parkinson’s Excellence Network is the driving force for improving Parkinson’s care, connecting and equipping professionals to provide the services people affected by the condition want to see.
The tools, education and data it provides are crucial for better services and professional development.
The network links key professionals and people affected by Parkinson’s, bringing new opportunities to learn from each other and work together for change.
Visit the UK Parkinson's Excellence Network