Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Monday, 6 May 2024, 8:03 PM
The problem of antimicrobial resistance
Introduction
Welcome to The problem of antimicrobial resistance, an introductory module aimed at all learners. Many basic concepts are introduced in this module and will be discussed in more detail in other modules.
The problem of antimicrobial resistance will take you back in time to gain insight into our understanding of prevention and treatment of infectious diseases. You will learn about hygiene theory, germ theory and the discovery of antibiotics. You will gain an understanding of the importance of antibiotics in modern medicine and other aspects of modern life. Unfortunately, the antibiotics that we have today are becoming less effective in the treatment of infections due to antimicrobial resistance. This is a very big threat to global health. You will learn how resistance develops in bacteria and what the drivers are. In the final section you will learn about ongoing global efforts to tackle the problem of antimicrobial resistance (AMR), and finally reflect on what you can do to bring about change.
By the end of this module, you should be able to:
- define the term ‘antibiotic’ and describe the importance of antibiotics in modern society
- explain how the overuse and misuse of antibiotics contribute to bacterial resistance
- describe the scale and nature of antibiotic resistance worldwide, and discuss the consequences of a future without antibiotics
- explain why the problem of AMR needs a One Health approach
- reflect on your own role and those of your colleagues in tackling the AMR crisis.
Activity 1: Assessing your skills and knowledge
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 Life before antibiotics
The problem of antimicrobial resistance (AMR) is a universal issue that we could all be directly or indirectly impacted by. AMR was responsible for an estimated 700,000 deaths worldwide in 2016; if we don’t act now, this number is likely to increase in the future.
This section provides an historic perspective on important developments that have shaped modern medicine and provides insights to understanding the problem of AMR that we face today.
After completing this section you will:
- understand how concepts related to the prevention, causes and treatment of infectious diseases developed over time
- understand the importance of good hygiene measures in preventing infections
- understand the importance of antibiotics in modern medicine and modern life
- appreciate that the pre-antibiotic era is not that long ago.
1.1 Medicine in the 1800s
In order to better understand some key aspects of modern medicine, we first go back to a time when we did not know much about infectious diseases; a time when an infection that can now be easily treated and cured could kill you.
Activity 2: Living and dying in the 18th century
Watch Video 1, about the death of George Washington in 1799. As you watch, keep the following questions in mind:
- What did doctors think caused the disease?
- What treatment was given to George Washington?
- What might have caused this disease?
- What kind of treatments are available today?

Transcript: Video 1
Answer
- What did doctors think caused the disease?
- His humours were unbalanced, or perhaps he breathed in miasma (foul air).
- What treatment was given to George Washington?
- They drained his blood (blood-letting).
- They applied ground-up green beetle on his throat.
- What do we now know about what might have caused this disease?
- Microscopic organisms can cause
infectious diseases , such as the one that attacked George Washington’s throat. Other examples of diseases caused by microbes are bubonic plague, tuberculosis and gangrene.
- Microscopic organisms can cause
- What kind of treatments are available today for management of infections caused by microbes?
- We now know that infections are caused by microscopic organisms (microbes), including bacteria. Antibiotics are used to treat infections caused by bacteria. (You will learn more about antibiotics in the next section.)
1.2 Discoveries in modern medicine
A lot has changed since the 1800s: our understanding of the causes, treatment and prevention of infectious diseases has considerably improved. In this section you will learn how our knowledge developed, who contributed to developing new insights and why this new approach was so revolutionary at the time.
Activity 3: Development of hygiene theory
Watch Video 2 on the discoveries of Ignaz Semmelweis, a physician working in Vienna in the mid-19th century, and answer the question that follows.
Transcript: Video 2
Years before the germ theory of disease was developed, the Viennese physician Ignaz Semmelweis came to the startling discovery that doctors could save the lives of patients simply by washing their hands. Although he couldn’t explain why hand disinfection was so effective, his research in 1847 would go on to transform the way surgery is carried out and infectious diseases are controlled today.
Known locally as the saviour of women, Semmelweis initiated handwashing policies which helped to drastically reduce the mortality rate from childbed fever in Viennese obstetrics clinics where he worked.
Childbed fever, or puerperal fever, is the infection of the female reproductive organs following childbirth and was a common cause of death at the time. Semmelweis’ attention was first drawn to the mortality rates at the clinics he worked at, because women would beg to be admitted to the clinics run by midwives rather than those staffed by students. The women were so desperate to avoid the student clinics that they would rather give birth in the street. After looking into the mortality rates of the two clinics, he found that the student run clinic did indeed have a much higher mortality rate for puerperal fever – sometimes three times higher. After eliminating various factors, Semmelweis came to the conclusion that the students carried something from the dissection rooms where they performed autopsies, to the patients they examined during labor. He ordered the students to wash their hands in a solution of chlorinated lime before each examination. The mortality rate fell from 18 per cent to 1 per cent.
Despite the success of this policy, Semmelweis faced the wrath of the medical community. He could offer no acceptable explanation for his findings as the notion that there were germs causing disease was unaccepted, and various doctors were offended by the insinuation that their hands were dirty. He was ostracised and ridiculed by the leading medical figures of his time, and eventually driven out of Vienna.
After struggling for years to promote his hand disinfection policies, Semmelweis was admitted to an insane asylum at the age of 47. He died 14 days later.
What did Ignaz Semmelweis discover?
Answer
He discovered that doctors could save lives by simply washing their hands.
Semmelweis was not the first person to see the connection between hygiene and the spread of puerperal fever (childbirth fever); Alexander Gordon had made a similar observation about 50 years earlier. He discovered that puerperal fever was spread from patient to patient by the attending midwife or doctor. To limit the spread of the disease, he recommended fumigating clothing and burning bedlinen used by women with puerperal fever. He also recommended the cleanliness of the attending doctors and midwives.
At the time that Gordon and later Semmelweis made their discoveries on the importance of hygiene in the spread of disease, they did not know what actually caused infections. That discovery was made a few years later by the collective efforts of Louis Pasteur and Robert Koch, whose discovered that diseases were caused by microscopic organisms – which they called ‘germs’.
Activity 4: Definitions of microbes
a.
True
b.
False
The correct answer is a.
Discussion
When
a.
True
b.
False
The correct answer is b.
Discussion
Microbes are microscopic organisms that exist as a single cell or as a colony of cells. Some microbes cause diseases; these microbes are called pathogens (they are
a.
True
b.
False
The correct answer is a.
Discussion
Microbes are microscopic organisms that exist as a single cell or as a colony of cells. Bacteria are cellular micro-organisms. Some bacteria cause diseases and are referred to as pathogenic bacteria. Most species of bacteria, however, are not harmful to us. You will learn more about bacteria in Section 2.1.
a.
True
b.
False
The correct answer is b.
Discussion
Yeasts, and single-celled animals such as amoebae, are also classed as microbes. They may or may not be pathogenic.
Discovery of antibiotics
After germ theory was established, people knew what caused infectious diseases. The search for a cure for these diseases had begun.
In 1928, Alexander Fleming discovered which
Transcript: Video 3
In Section 2.2 you will learn more about what antibiotics are, and in Section 2.3 you will learn more about what antibiotics do.
Timeline
The following timeline summarises important moments in history that shaped our understanding of the prevention, causes and treatment of infectious diseases:
Before 1800: The general understanding was that diseases were caused by bad gases, which were called miasmas, or by an imbalance of fluids in the body. The treatment for diseases at that time included blood-letting and treatments that are now regarded as quackery.
1795: Alexander Gordon observed that puerperal fever was spread between patients by attending midwives and doctors. He recommended cleanliness of the attending medical staff as a measure to prevent disease from spreading, but his recommendations were largely ignored.
1847: Ignaz Semmelweis discovered hygiene theory – that is, the importance of handwashing in preventing puerperal fever. He was also largely ignored.
1854: John Snow traced an outbreak of cholera to a single contaminated water pump in London, and thus discovered the link between contaminated water and this disease. He is considered to be one of the founding fathers of the discipline of
1850–1880: Collective efforts by Louis Pasteur and Robert Koch led to the discovery that diseases are caused by micro-organisms (germ theory).
1928: Alexander Fleming discovered that a substance produced by a mould – which he called penicillin – could kill bacteria. Florey and Chain later played an important role in making the antibiotic penicillin widely available. Fleming, Florey and Chain were awarded the 1945 Nobel Prize for Medicine for their achievements.
2 The golden age of medicine
In this section you will learn about antibiotics: what they are, how they work and the important role they play in modern medicine but also modern life. Here, you will be introduced to the basic concepts that will be explained in more detail in other modules in this course.
After this section you will be able to:
- define the term ‘antibiotic’ and give examples
- describe how antibiotics work
- describe the role of antibiotics in modern medicine and modern life.
2.1 What bacteria are
Bacteria are the most numerous organisms living on Earth. They live all around us: in our gut, in the soil and in water. Bacteria are pretty much all over the place.
Activity 5: Bacterial growth
The secret of the success of bacteria – why there are so many of them – is that they can reproduce rapidly. Under the right conditions, some types of bacteria can replicate every 20 minutes. Take a look at Video 4 to see what this looks like, and answer the question that follows.
Transcript: Video 4
The video mentions that a bacterial infection can take hold so quickly because bacteria can double their numbers in such a short time. What are the three options mentioned in the video that help to stop the growth and replication cycle of bacteria?
Answer
The three options are:
- handwashing with soap
- vaccination to boost the immune system
- antibacterial drugs.
Most bacteria do not cause us any harm. However, a small group of bacteria – about 500 species – can cause diseases in humans, animals and/or plants. These bacteria are called pathogenic, and they are capable of causing an infection by evading the host’s normal defences and invading cells or tissues. They may also produce harmful toxins (poisons).
Many bacteria are
Activity 6: Diseases caused by bacteria in humans, animals and plants
Think about examples of diseases in humans, plants and animals that are caused by bacteria.
Answer
Diseases caused by bacteria in humans:
- Tuberculosis (TB) is caused by Mycobacterium tuberculosis and mainly affects the lungs. The classic symptom of active tuberculosis is a chronic cough with bloodstained sputum, fever and weight loss. This disease can spread to other people through respiratory secretions when patients with active TB cough, spit up their sputum or sneeze.
- Tetanus, also known as lockjaw, is characterised by muscle cramps and is caused by Clostridium tetani. This bacterium is commonly found in soil, dusts and manure. The bacteria can enter the body through a break in the skin and produce toxins that cause the muscle contractions. This disease is not spread from human to human.
- Typhoid fever is caused by Salmonella typhi serovar Typhi, sometimes abbreviated as Salmonella Typhi. Patients infected with it may experience mild to severe symptoms including fever, abdominal pain constipation and vomiting. This disease is spread through contaminated water or food.
- Pneumonia is an infection of the lungs. Some bacterial strains that can cause pneumonia include Streptococcus pneumoniae, Staphylococcus aureus and Klebsiella pneumoniae. Bacteria are the most common cause of pneumonia; viruses cause only about one third of cases of pneumonia in adults.
Diseases caused by bacteria in animals:
- Anthrax in cattle, sheep and goats is usually spread by contact with spores of Bacillus anthracis in the soil or from pastures. Animals present with weakness, staggering and bloody discharges, often rapidly leading to death – although sometimes sudden death of livestock is the first sign of the disease. Anthrax is a
zoonotic disease, so it can also be passed from animals to humans. - Salmonellosis in poultry, with Salmonella species causing disease in a wide range of birds including chickens and turkeys. Different species and serovars present differently, with common presentations being pullorum disease, fowl typhoid and fowl paratyphoid. Some strains of Salmonella in poultry can be passed to humans through the food chain.
- There are several species of Brucella which tend to present slightly differently in different species. The most common sign of brucellosis in livestock is abortion during the final trimester of pregnancy. Some species of Brucella are zoonotic.
- One of the most common aquatic diseases is Vibrio, which leads to massive mortality of cultured shrimp, fish and shellfish. Vibriosis (Vibrio illness) can be zoonotic, and Vibrio cholerae can cause cholera in humans.
Diseases caused by bacteria in plants:
- Bacterial leaf streak and black chaff are a major bacterial disease of wheat. The bacteria (Xanthomonas translucens) are seed-borne. Typical symptoms on the leaf consist of elongated, light brown lesions, several centimetres long, that are initially distinct but later coalesce to cover larger solid areas. Yield losses can be as high as 40%.
- Epidemics of rice bacterial blight caused by Xanthomonas oryzae have been observed in Asia, the western coast of Africa, Australia and Latin America. The early signs consist of streaks that spread from leaf tips and margins, eventually oozing a milky substance that dries into yellow droplets. Later on, leaves will die; infected seedlings die within a few weeks of infection.
- Bacterial canker mainly affects tomatoes (caused by the bacterium Clavibacter michiganensis), present worldwide. Infections result in wilting, defoliation, desiccation, skin cankers, significantly reduced fruit yield and quality, and ultimately plant death.
2.2 What are antibiotics?
Activity 7: What are antibiotics?
Watch Video 5, on antibiotics:

Transcript: Video 5
Now write down in your own words a definition of antibiotics.
Discussion
Compounds that are made by microbes to fight other microbes are naturally occurring antibiotics. Scientists have copied and often modified these compounds to use to fight bacteria in humans, animals and plants.
Activity 8: Study definitions
In this course you have already seen a few concepts, and you have read about microbes, bacteria, antibiotics and antimicrobials. Take a few minutes to study the definitions listed in the table below and then answer the questions that follow.
| Term | Definition |
|---|---|
| Antibacterial | Compounds that kill or inhibit the growth of bacteria. |
| Antibiotic | Compounds that kill or inhibit the growth of microbes/micro-organisms. ‘An antibiotic is an agent or substance that is produced by or derived from a micro-organism that kills or inhibits the growth of another living micro-organism. Antibiotic substances that are synthetic, semi-synthetic, or derived from plants or animals are, strictly speaking, not antibiotics. However, for the purposes of this module they are included. In this document “antibiotic” refers to an antimicrobial agent with the ability to kill or inhibit bacterial growth.’ (WHO, 2019) |
| Antifungal | Compounds that kill or inhibit the growth of fungi. |
| Antimicrobial | Compounds that kill or inhibit the growth of microbes/micro-organisms. ‘An antimicrobial is an agent or substance derived from any source (micro-organisms, plants, animals, synthetic or semi-synthetic) that acts against any type of micro-organism, such as bacteria (antibacterial), mycobacteria (anti-mycobacterial), fungi (antifungal), parasite (anti-parasitic) and viruses (antiviral).’ (WHO, 2019) |
| Antiparasitic | Compounds that kill or inhibit the growth of parasites. |
| Antiviral | Compounds that kill or inhibit the growth of viruses. |
a.
True
b.
False
The correct answer is a.
Answer
Antibacterials are a kind of antimicrobial.
a.
True
b.
False
The correct answer is b.
Answer
Antimicrobials also include antifungals, antivirals and antiparasitics.
Note: because this course mostly focuses on bacterial antimicrobial resistance, we will generally use the terms ‘antibiotic’ and ‘antimicrobial’ to mean ‘antibacterial’.
2.3 What antibiotics do
Antibiotics that are used for medical treatment work through a mechanism of
That seems very obvious now, but let’s briefly go back in time again. Not even that long ago – let’s say about 100 years …
Do you remember the year that antibiotics were discovered?
1928.
At that time there was some understanding about the importance of hygiene measures to prevent spread of infectious disease. However, infections were difficult to treat, because the treatments that were available not only killed the infection, but also often killed the patient.
The discovery of
Not all antibiotics attack bacteria in the same way: some attack the cell wall, some attack bacterial protein syntheses and some attack the DNA-replicating mechanism of bacteria. In all cases, they tackle structures or biochemical processes that are either not found in human or animal cells, or don’t work in the same way. Antibiotics are classified based on their chemical structure: those with a similar chemical structure tend to have similar antibacterial activity. (You will learn more about this in Introducing antimicrobial resistance.)
Some classes of antibiotics target several bacterial species: these are called
Activity 9: The antibiotics quiz
a.
bacteria and viruses
b.
bacteria
c.
viruses
The correct answer is b.
Discussion
The term ‘antibiotic’ describes an antimicrobial agent produced by a microbe to protect it against other microbes, or a drug designed (often with a natural product as a template) to target pathogenic/harmful organisms. However, the term ‘antibiotic’ is widely used to mean ‘antibacterial’ (any compound used to kill, or inhibit the growth of, bacteria).
Using this definition, antibiotics specifically target bacteria. They are not effective against infections caused by viruses (such as influenza), or the viruses that cause the common cold – although they may sometimes be used against secondary bacterial infections that can follow viral infections. Indiscriminate use of antibiotics to treat viral infections leads to unnecessary and inappropriate use of antibiotics and antimicrobial resistance.
a.
do not cause side-effects
b.
are only active against pathogens
c.
can be used in a prophylactic manner to prevent bacterial infections
d.
stimulate the body’s immune system.
The correct answer is c.
Discussion
Antibiotics can be used in a prophylactic manner to prevent bacterial infections. This happens during some surgical procedures.
Antibiotics are not selective; they inhibit or kill ‘good’ bacteria along with ‘bad’ bacteria. This can lead to common side-effects such as an upset stomach and antibiotic-associated diarrhoea caused by Clostridium difficile or other gut microbes. Antibiotics do not enhance the body’s immune response.
2.4 Role of antibiotics in modern medicine
In the previous sections you learned that:
- pathogenic bacteria can cause infection in humans, animals and plants
- antibiotics are used to treat bacterial infections.
However, antibiotics can be used in other scenarios, beyond the treatment of infectious diseases.
Activity 10: Antibiotics in modern medicine
Based on the understanding that you have now, reflect on the role that antibiotics play in these different aspects of modern medicine:
- The use of antibiotics in cancer treatment.
Discussion
Cancer is a disease caused by cells that grow excessively, creating tumours and potentially invading other tissues. Chemotherapy is one of the principal treatments for cancer; a major-side effect is that the immune system is weakened. In Section 2.1 you learned that opportunistic bacteria can take advantage of this situation and cause an infection. Antibiotics are important to treat bacterial infections in patients with extremely weakened immune systems due to chemotherapy.
- The use of antibiotics for
surgical prophylaxis .
Discussion
Antibiotics are used before certain surgical procedures to prevent infection. For example, for orthopaedic operations where implants are put in to replace joints (such as a hip or knee), there is a possibility of micro-organisms infecting the prosthesis.
Nowadays, surgery is performed in aseptic conditions to minimise the risk of infection. Without antibiotics, surgery would still be possible; however, the risk of an infection as a result of the surgery would be bigger and more difficult to treat. Surgery was performed before the discovery of antibiotics, but mortality rates were very high.
2.5 The role of antibiotics in food production
In the previous sections you learned that:
- pathogenic bacteria can cause infection in humans, animals and plants
- antibiotics are used to treat bacterial infections
- antibiotics are also used to prevent infections in patients with weakened immune systems, for example in cancer patients
Activity 11: The role of antibiotics beyond human medicine
Based on the understanding that you have now, reflect on the role that antibiotics play beyond human medicine.
What is the role of antibiotics in the production of crops?
Discussion
Bacteria and fungi cause significant plant disease and production losses worldwide. Pesticides, including antimicrobial pesticides, play an important role in reducing losses in crop production. Some of the same drugs that are used in human and veterinary medicine – for example, streptomycin, tetracyclines or triazoles – are also used to control plant diseases (FAO, 2018).
What is the role of antibiotics in food-animal production and veterinary medicine?
Discussion
In food-animal production, antibiotics are used to treat disease, prevent disease and promote growth. The rising global demand for animal-sourced food has led to more intensive, large-scale farming, with animals reared in confined spaces – often at a high density. These production systems promote pathogen spread. In some countries and production systems, prophylactic use of antibiotics to address disease problems in intensive systems is common. In other countries, industries have worked particularly hard on improving biosecurity to minimise or even completely eliminate the prophylactic use of antibiotics in food-animal production.
The use of antibiotics to promote growth in food-producing animals was common historically but is now considered inappropriate. Members of the World Organisation for Animal Health (OIE) agreed in 2016 to ‘phasing out the use of antibiotics for growth promotion in the absence of risk analysis’. The latest OIE Annual Report on Antimicrobial Agents Intended for Use in Animals reported that 77% of the 118 nations that submitted data on antimicrobial use in animals responded that antimicrobials were not used for growth promotion in animals in their countries (OIE, 2020).
There is now a global consensus that the use of antibiotics for prophylaxis and growth promotion in animals should be minimised and avoided where possible. However, like humans, both food-producing and companion animals (e.g. cats, dogs and other pets) suffer from bacterial diseases that can easily be treated with antibiotics. Therefore, there will always be a role for treating individual animals therapeutically with antimicrobials. Failing to treat these animals results in unnecessary suffering (and in some cases, death), which threatens the livelihood of farmers and the welfare of animals.
There is a series of principles that can help reduce the impact of the necessary antibiotic use in animals on AMR in humans (e.g. avoiding the use of some antibiotics in animals that should be reserved for treatment of some bacterial infections in humans). These principles will be covered in the Antimicrobial stewardship in veterinary practice module
3 The problem of antibiotic resistance
In this section you will learn about antibiotic resistance and reflect on its implications for modern medicine and other aspects of our lives. In the previous section you learned about the importance of antibiotics today. In this section you will reflect on what a future without effective antibiotics would look like. You will also study figures and maps to get an understanding of the global scale of the problem of antibiotic resistance.
After this section you will:
- be able to define the term antibiotic resistance
- understand the scale of the problem of antibiotic resistance
- be able to reflect on a future without functional antibiotics.
3.1 Definition of resistance
Activity 12: A recap on definitions
Recall the definitions of:
- antimicrobial
- antibiotic
- antibacterial.
Discussion
The definitions are as follows:
| Term | Definition |
|---|---|
| Antibacterial | Compounds that kill or inhibit the growth of bacteria. |
| Antibiotic | Compounds that kill or inhibit the growth of microbes/micro-organisms. ‘An antibiotic is an agent or substance that is produced by or derived from a micro-organism that kills or inhibits the growth of another living micro-organism. Antibiotic substances that are synthetic, semi-synthetic, or derived from plants or animals are, strictly speaking, not antibiotics. However, for the purposes of this module they are included. In this document “antibiotic” refers to an antimicrobial agent with the ability to kill or inhibit bacterial growth.’ (WHO, 2019) |
| Antimicrobial | Compounds that kill or inhibit the growth of microbes/micro-organisms. ‘An antimicrobial is an agent or substance derived from any source (micro-organisms, plants, animals, synthetic or semi-synthetic) that acts against any type of micro-organism, such as bacteria (antibacterial), mycobacteria (anti-mycobacterial), fungi (antifungal), parasite (anti-parasitic) and viruses (antiviral).’ (WHO, 2019) |
Activity 13: Understanding resistance
Watch the following video on antibiotic resistance, up to 2:38. Don’t worry if you hear some terms in this video that you are unfamiliar with; it is important that you understand the overall concept of resistance to antibiotics in bacteria.
Transcript: Video 6
[MUSIC PLAYING]
In your own words, what is antibiotic resistance?
Discussion
Bacteria have developed ways to defend themselves against the antibiotics that other microbes produce. When bacteria can withstand attack by the antibiotic, they are termed resistant to that antibiotic.
Bacteria can be innately resistant or acquire resistance. (You will learn more about this in the module Introducing antimicrobial resistance.) The first,
Resistance can also be acquired. The two major ways of developing
Remember: bacteria are the ones that get resistant to antibiotics – not people, animals or plants.
The term ‘
In Section 4 we will look at the development and drivers of resistance in more detail, but first let’s take a moment to think about what this means for modern medicine (human and veterinary), and agriculture at the global scale.
3.2 Consequences of resistance
Activity 14: Consequences of resistance
In Sections 2.4 and 2.5 you learned about the role of antibiotics in modern medicine (human and veterinary), and agriculture.
Due to development of resistance in some bacteria, the antibiotics that we have available today are not always effective. Take a moment to reflect on what that means for:
- treatment of infectious diseases caused by bacteria
- surgery
- crop production
- meat production.
Discussion
Treatment of infectious diseases caused by bacteria
Antibiotics are the ‘magic bullets’ that can attack bacterial infections without killing the patients. That does not mean that the antibiotic treatment is completely harmless to the patients: antibiotics disrupt the normal bacterial flora as they not only kill the pathogenic organism, they also kill the normal flora (good bacteria). So antibiotics must be used carefully, only where appropriate, and should not be prescribed indiscriminately. For example, for a mild sore throat with no signs of fever, the infection is likely to be caused by a virus. Warm saline gargles may be effective at relieving symptoms, while antibiotics may have no effect at all. Similarly, in animals, small wounds can be managed with proper wound dressings and care without the need to prescribe antibiotics.
You have also learned that there are different classes of antibiotics that work in different ways. So if a bacterium becomes resistant to one kind of antibiotic, there might be another type of antibiotic with a different mechanism of action that is still effective against that bacterial infection. However, what happens if the bacteria become resistant to that one as well, and then to the next one and the next one? This is already happening with some bacteria, which we call
Surgery
Without effective antibiotics, surgery will become riskier because there will be no way to treat bacterial infections that might happen as a complication of surgery. To prevent the risk of infection, surgery should be performed in aseptic conditions. However, there is still a possibility that bacteria on patients’ skin may enter the body during the course of surgery. In Section 2.1 you saw that under the right conditions, bacteria can replicate very quickly. Without antibiotics to treat the infection, the risk of complications from the surgery is high.
Crop production
Bacteria and fungi cause significant plant disease and production losses worldwide. We need antibiotics to treat crop diseases caused by bacterial pathogens to preserve food security and livelihoods. Intensive agriculture often involves monocultures of crops in which pathogens can spread easily. In a plot of land where plants are widely spaced, plant diseases cannot spread as easily. The reality is that we are growing our crops in conditions that favour spread of pathogens, so we need to be able to continue to treat the diseases they cause.
Meat production
Antimicrobials are used to treat infections in food-producing animals. Just like humans, food-producing animals can be infected with bacterial pathogens. Because of the pressure placed by human population growth on agricultural systems, food-producing animals are often housed very close together in what’s referred to as intensive farming systems. When animals are housed close together, bacterial infections occur more frequently and spread more readily. Improving biosecurity in intensive farming systems can reduce the incidence of bacterial diseases but many of these farming systems may still require antimicrobials to control disease at some point so that animals do not suffer, and farms remain economically viable.
A good example is the use of antimicrobials to treat mastitis in high-producing dairy systems. In these systems, dairy cows are kept together in a large herd (possibly at high density), and milked at least twice daily using machines that attach to the cows’ teats. Milking with shared machinery and close proximity with other members of the herd can lead to infection of the mammary glands – mastitis, which is painful and reduces milk quality and safety.
There are many ways to reduce and control the incidence of mastitis on a dairy farm, such as making sure milking equipment is cleaned properly and that cows’ teats are cleaned before and after milking. Despite this, most high-producing commercial farms still need to treat mastitic cows with antimicrobials when necessary. If these antimicrobials become ineffective, treatment of cows with mastitis will also be less effective. This will likely lead to more euthanasia of infected cows and will impact the quality, quantity and expense of milk production.
If we lose functioning antibiotics, will we go back to life as it was in the 1800s? (Use the theory you learned in Section 1 of this module.)
Discussion
No. Because of the developments in hygiene theory and epidemiology (starting with the work of John Snow), we know much more about the spread of disease in the population and how to prevent it.
Take for example the way we prevent and treat cholera. Cholera is an infection of the small intestine by a bacterium called Vibrio cholerae. The classic symptom is a watery diarrhoea that quickly leads to severe dehydration and electrolyte imbalance. In 1854, John Snow discovered the link between contaminated water and cholera, as you saw in Section 1.2. He was able to convince the local council that the water well was the source of the outbreak. The outbreak was controlled by removing the well pump’s handle to disable it, thus preventing people from drinking the contaminated water.
Another important advance that has been made in cholera prevention is the development of cholera vaccines. However, despite preventative measures, there are still many people getting sick from this disease. Antibiotics are available to treat it, but that is not the only possible treatment. Oral rehydration therapy is now recommended as the first line of treatment for cholera, and this alone will successfully treat most cases. The WHO recommends prescription of antibiotics only to those with the most severe infections.
3.3 Scale of resistance
Although we would not go back to life as it was in the 19th century if we lost antibiotics, we should definitely not underestimate the impact that losing them would have on global health.
In 2016, the UN assembly gathered to discuss the problem of AMR and called it one of the biggest threats to global health. This was only the fourth time in the history of the UN that a health topic was discussed at its General Assembly – the other three were HIV, noncommunicable diseases and Ebola (WHO, 2016).
Activity 15: Resistance of Acinetobacter baumannii to carbapenems
Now let’s look at the scale of resistance worldwide.
Acinetobacter baumannii is an opportunistic pathogen that can cause a range of diseases, including pneumonia and bloodstream infections. Carbapenems are a class of highly effective broad-spectrum antibiotics, usually reserved for treating multi-drug-resistant infections. The fact that this species has developed resistance to such an effective and important antibiotic class is particularly worrying.
In Figure 2 you see the resistance that Acinetobacter baumannii has developed against a class of antibiotic known as carbapenems as documented by the Center for Disease Dynamics, Economics & Policy (CDDEP). The percentage of resistant isolates of Acinetobacter baumannii is indicated in blue; the darker the country, the higher the percentage of resistance. There are no data available on the countries in light grey.
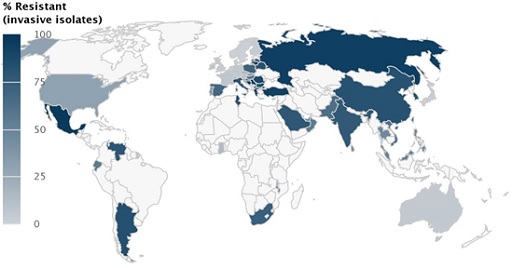
Is data available from your country?
Which countries have the highest level of resistant isolates of Acinetobacter baumannii against carbapenems?
Discussion
The darker blue the country, the higher the proportion of isolates that were found to be resistant. High levels of resistance are found in Mexico, Venezuela, Argentina, South Africa, Tunisia, Poland, Ukraine, Spain, Italy, Greece, Turkey, Saudi Arabia, Russia, China, India and Pakistan.
Let’s zoom in to a few countries to see the percentage of Acinetobacter baumannii isolates found to be resistant over time. Take a minute to look at the trends of resistance in Russia, the United States and Venezuela (Figures 3–5).
The figures show the percentage of Acinetobacter baumannii isolates resistant to carbapenems over time in Russia, Venezuela and the United States in different years as documented by the CDDEP. Data are based on different data sources.
Different colours on the maps are assigned at random.
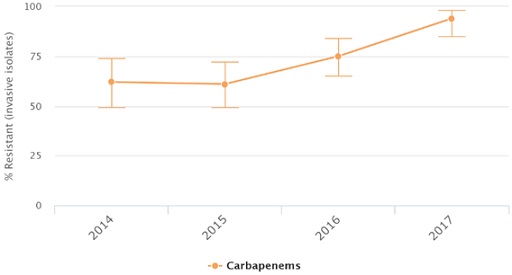
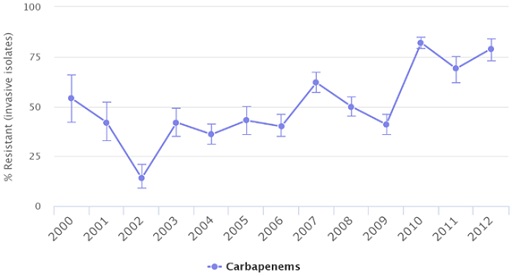

What is the trend that you can identify on the percentage of Acinetobacter baumannii isolates resistant to carbapenems over time? What is different in the trend in the United States?
Discussion
In Russia and Venezuela, the level of resistance has increased over time. It is not increasing in a straight line, but the trend is going up. In the United States, the level of resistance increased until 2009 but started decreasing after that year.
Why are the timelines of the graphs different?
Discussion
The timelines of the graphs are different due to the practical reasons of the availability of information. In Section 5.3 you will learn about international efforts to standardise data collection on the levels of resistance of different pathogens all over the world.
Unfortunately, Acinetobacter baumannii is not the only type of bacterium that shows resistance to this class of antibiotics: several other types of bacteria have developed resistance to carbapenems or other broad-spectrum antibiotics such as cephalosporins and vancomycin. The WHO developed the priority pathogen list (see Table 1) for development of new antibiotics. This list (adapted from WHO, 2017) ranks antibiotic-resistant bacterial pathogens for which alternative treatments are urgently required, giving each a priority rating. As you can see, the priority rating for Acinetobacter baumannii is ‘critical’.
| Bacterium | Type of antibiotic resistance | Priority rating |
|---|---|---|
| Acinetobacter baumannii | Carbapenem | Critical |
| Pseudomonas aeruginosa | Carbapenem | Critical |
| Enterobacteriaceae | Carbapenem, cephalosporins | Critical |
| Enterococcus faecium | Vancomycin | High |
| Staphylococcus aureus | Vancomycin, methicillin | High |
| Helicobacter pylori | Clarithromycin | High |
| Campylobacter sp. | Fluoroquinolone | High |
| Salmonella sp. | Fluoroquinolone | High |
| Neisseria gonorrhoeae | Cephalosporin, fluoroquinolone | High |
| Streptococcus pneumoniae | Penicillin | Medium |
| Haemophilus influenzae | Ampicillin | Medium |
| Shigella sp. | Fluroquinolone | Medium |
The WHO has also produced a list of priority pathogens for surveillance as part of its Global Antimicrobial Resistance Surveillance (GLASS) programme. This is a list of organisms in which high levels of resistance have been reported and are also some of the most common causes of bacterial infections. The priority pathogens for the GLASS surveillance (WHO, 2015) are:
- Acinetobacter baumannii
- Escherichia coli
- Klebsiella pneumoniae
- Neisseria gonorrhoeae
- Salmonella sp.
- Staphylococcus aureus
- Streptococcus pneumoniae.
As you may have noticed, there is a lot of overlap between the two lists.
4 How resistance develops
In the previous section you learned about the global scale and impact of AMR on modern life. In this section you will learn about the causes that contribute to this problem – specifically, the development and drivers of AMR.
After this section you will understand:
- that development of antibiotic resistance is a naturally occurring process that protects bacteria
- that increased use of antibiotics has sped up the natural process of resistance development
- how resistant bacteria spread in the environment
- that use of antimicrobials in different sectors is a driver for AMR.
4.1 Development of resistance
In Section 2.2 you learned that antibiotics occur naturally, although scientists have copied and modified those compounds to produce synthetic antibiotics, and most antibiotics in current use are not naturally produced. Just as the development of antibiotics is a naturally occurring process, so too is the development of resistance to antibiotics.
Activity 16: Antibiotic resistance occurs in nature
Watch this video about bacteria living very remotely from humans in a resource-scarce environment.

Transcript: Video 7
4.2 Spread of resistance in bacteria and the environment
Earlier in this module you learned that bacteria are all around us. Some types of bacteria are common to all living beings (humans, animals and plants). Remember from Section 2.1 how fast bacteria replicate?
Consider a few Salmonella bacteria that have infected a chicken. The chicken is treated with antibiotics, and one of the bacteria happens to be resistant to the antibiotic that is given. All the other bacteria will be killed by the antibiotic, but the resistant bacterium will survive, divide, multiply and pass the resistance to all offspring. In addition, bacteria can pass on resistance between each other by other mechanisms called horizontal transfer, which is explained in the module Introducing antibiotic resistance.
Activity 17: Spread of antibiotics in the environment
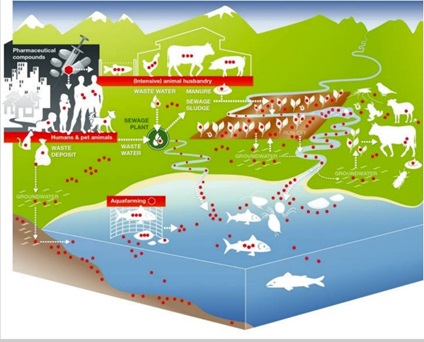
Study Figure 6 (from Berkner et al., 2014) on the different ways that antibiotics spread in the environment and answer the question that follows. Just as antibiotics can spread in the environment, so can some resistant bacteria.
Identify three ways that antibiotics can end up in the groundwater.
Discussion
Three ways that antibiotics can end up in the groundwater are through:
- waste deposits from humans that contain antibiotic-resistant bacteria
- run-off from fields that have been fertilised with manure that carries antibiotic-resistant bacteria, or with sewage sludge from the sewage plant that carries antibiotic-resistant bacteria
- excrement or droppings from animals that carry antibiotic-resistant bacteria.
4.3 Drivers of resistance
Activity 18: Resistance to newly discovered antibiotics
Listen to part of a TED talk (between 3:49 and 5:00 in Video 8) and pay particular attention to the figure that appears onscreen at around 4:45, which summarises the development of resistance against different classes of antibiotics.
Transcript: Video 8
Briefly, it works like this. Bacteria compete against each other, for resources, for food, by manufacturing lethal compounds that they direct against each other. Other bacteria to protect themselves, evolve defences against that chemical attack.
When we first made antibiotics, we took those compounds into the lab, and made our own versions of them. And bacteria responded to our attack, the way they always had.
Here is what happened next:
Penicillin was distributed in 1943, and widespread penicillin resistance arrived by 1945.
Vancomycin arrived in 1972; vancomycin resistance in 1988.
Imipenem in 1985 and resistance to it in 1998.
Daptomycin, one of the most recent drugs, in 2003, and resistance to it just a year later in 2004.
For 70 years, we played a game of leapfrog: our drug, and then resistance. And then another drug, and then resistance again.
The more antibiotics we use, the more we select for resistance. Any
This process holds true for any newly discovered antibiotic. Evolution means that resistance will inevitably emerge, but it will do so more rapidly if we do not use antibiotics with care. Let’s look at some graphs on
Activity 19: Human antimicrobial consumption
Study Figure 7 and answer the questions that follow. It shows the percentage change in antibiotic consumption per capita between 2000 and 2010. Percentage decrease is indicated in blue while percentage increase is indicated in red. Lower percentage changes are indicated by lighter colours. No data are available for the countries shown in grey.
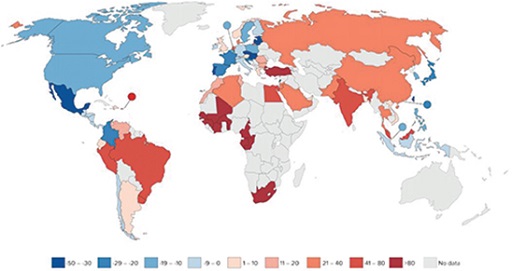
- Is human antibiotic consumption data available for the country that you live in?
- What global trend(s) can you identify in human antibiotic consumption?
Discussion
In some countries the consumption of antibiotics by humans has decreased between 2000 and 2010, and in others it has increased. Decrease of antibiotic consumption is seen in the Americas, most countries in Europe and in eastern Asian countries. Increase in antibiotic consumption is seen in South America, Africa and Asia.
Generally, based on Figure 7, you can say that in high-income countries the antibiotic consumption by humans has decreased between 2000 and 2010, whereas it has increased in low- and middle-income countries. However, no information is provided on the use of antibiotics, such as who they were given to and whether they were used appropriately. Understanding how antibiotics are used is another important factor in understanding the drivers of AMR.
Antibiotics are also used to keep animals healthy. This includes both companion animals and food-producing animals. Use of antimicrobials in companion animals is likely to have less impact on AMR than antimicrobial use in food-producing animals. Companion animal medicine is usually based on therapeutic treatment of a small number of individual animals, whereas antimicrobial use in food-producing animals sometimes occurs at a larger scale and may include use for prophylaxis or growth promotion. In addition, treatment of food-producing animals may result in resistant bacteria entering the food chain.
The Food and Agriculture Organization (FAO) states that estimates of the total use of antimicrobials in agriculture vary considerably. This is mainly due to a lack of systems in place to collect information on use of antimicrobials in animals, although in Section 5.3 you will learn that global efforts are being made to address this data gap. Antimicrobial use in livestock and in other food-producing animals such as aquatic animals is projected to increase over the coming decades based on the increased demand for animal-sourced food products (FAO).
Activity 20: AMR in aquaculture
Watch Video 9 (up to 0:50), which looks at AMR in aquaculture:
Transcript: Video 9
Why antimicrobial resistance (AMR) in aquaculture matters for the One Health approach.
Aquaculture is one of the fastest-growing food production sectors worldwide, especially in low- and middle-income countries. But as aquaculture intensifies to meet global demand, so does the disease burden affecting aquatic animals. Antibiotics are not a long-term solution and tend to be misused without proper training and consultation, often leading to resistant bacteria: a form of AMR. AMR refers to any microbe that develops resistance to the substance designed to control it.
Antimicrobials are given to food-producing animals. Drug-resistant bacteria develop in animals; drug-resistant bacteria can spread in the environment and food, and can be transferred to people by eating food.
Resistant bacteria is a major concern to human, animal and environmental health.
Why should we be concerned about using antimicrobials in (aquatic) animals? For inspiration for your answer, look at the figure that you studied in Activity 17.
The link between use of antimicrobials in food-producing animals and AMR spread among humans requires further study, because the link isn’t as clear as we think. There is some evidence that use in animals contributes to AMR spread in people, but what isn’t clear is how much it contributes compared to use in human health. Further studies are also required to quantify the volumes of antibiotics used in companion animals compared to food-producing animals. Although we might assume there are much higher volumes of antimicrobials used in food-producing animals, there are limited data available on this point, and even less on the reasons for use (treatment, prophylaxis, growth promotion). This data gap is slowly changing as countries start to improve the ways they measure and monitor the quantities of antibiotics and how they are used in different sectors.
In conclusion, global antibiotic consumption has increased since 2000 and is predicted to continue increase in the future (WHO, 2015b). In some countries, antibiotic use in humans and animals has decreased, but the opposite has happened in others. It is important to remember that any encounter a bacterial population has with an antibiotic may select for resistant bacteria. This is irrespective of whether bacteria are innately resistant or have become resistant due to mutation or gene transfer. The antibiotics kill the non-resistant bacteria, but the resistant bacteria survive. As described above, we don’t yet fully understand the links between use in agriculture and resistance in human disease. There are proven examples of resistant bacteria in animals transferring to humans through the environment and/or the food chain; however, there is a poor understanding of the scale of these events and their impact, and it is likely that, overall, AMR in humans is related to antimicrobial use both in healthcare and in farming. All sectors, therefore, need to address the issue of misuse of antibiotics to do their part in minimising the emergence and spread of AMR.
Activity 21: Reflection on use of antimicrobials
Should we just stop using antibiotics?
Discussion
No, of course not. Antibiotics are very important to us. We just need to make sure we use them responsibly in all sectors. Also, we should look at things that we can do to limit the use of antibiotics wherever possible. There are many things we can all do, as citizens and in our professional lives, to address the problem of AMR. In the next section you will learn about this.
5 The global response to fight AMR
In the previous section you learned about some of the underlying causes, the global scale and the impact of the problem of AMR. This section introduces steps we can take to address this problem, One Health and global efforts that are currently being made to address the problem of AMR.
After this section you will be able to:
- explain why the problem of AMR needs a One Health approach
- know about ongoing global efforts to address the problem of AMR
- reflect on your own role and those of your colleagues in tackling the problem of AMR.
5.1 What can we do to address the problem of AMR?
The problem of AMR is a universal issue; every one of us could be directly or indirectly impacted by it. AMR already has a significant impact, being responsible for an estimated 700,000 deaths worldwide in 2014 (Review on Antimicrobial Resistance, 2014), and this impact will become increasingly severe if action is not taken now.
In this section you will learn about steps we can and should take to fight the problem of AMR. You will use your understanding of the underlying causes that have led to the global scale of the problem of AMR to reflect on how all these steps are in themselves contributing to fighting it.
Activity 22: Ten things we can do to reduce AMR
Watch Video 10, which shows ten things we can do to reduce AMR.

Transcript: Video 10
Take a moment to reflect on these ten steps. Pick three steps and, use your understanding of the underlying causes and drivers of the problem of AMR to write down how each of these steps contributes to addressing the problem. If you have difficulty thinking of things you can do, just watch the video again.
Activity 23: Self-reflection
The AMR crisis affects us all so we can all do something to fight it.
- Think of three things you can do in your personal life that are important in combatting the AMR crisis.
- How can you contribute to combatting the AMR crisis in your professional life?
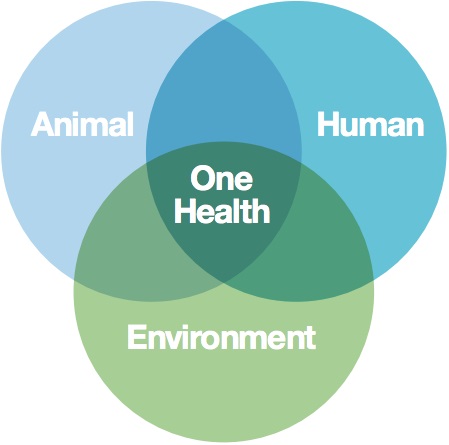
5.2 One Health
In AMR surveillance and you you were introduced to the concept of
In this module you have learned that antimicrobial-resistant bacteria can be found in humans, animals and the environment. It makes sense, therefore, to take a One Health approach to address the problem of AMR.
Activity 24: Self-reflection
- Look at Figure 8. In which area does your professional expertise fall?
- In which area(s) of Figure 8 do you lack professional expertise? Do you know professionals in your network who have this expertise? If not, how would you get in touch with professionals with this expertise?
5.3 International efforts
In Section 3.3 you learned that the UN General Assembly gathered in September 2016 to discuss the problem of AMR.
The 193 member states committed to work collaboratively to take worldwide action to control AMR by signing the UN Declaration on AMR. All the declaration signatories have agreed that drug-resistant infections must be tackled as a priority and have committed to:
- developing surveillance and regulatory systems on the use and sales of antimicrobial medicines for humans and animals
- encouraging innovative ways to develop new antibiotics and improve rapid diagnostics
- raising awareness among health professionals and the public on how to prevent drug-resistant infections.
By taking this course and discussing what you have learnt with colleagues, friends and family, you are already helping to raise awareness of AMR.
AMR surveillance is critical to tackling AMR, and is the backbone of these efforts. In AMR surveillance and you you learned that AMR surveillance is the ongoing collection, analysis, interpretation and dissemination of data related to AMR.
- At a global level, AMR surveillance provides quantitative data about the spread of resistant strains of bacteria, revealing trends and potentially identifying hotspots of resistant infections.
- At a regional level, surveillance data informs intervention priorities and helps to identify gaps in service delivery.
- At a national level, data guides planning and resource-allocation, and informs policies and responses to patterns and trends.
AMR surveillance data are important to inform policy-makers and decision makers at all levels.
Let’s look at two surveillance programs in the framework of the Global Action Plan on AMR supported by the tripartite collaboration:
Use of antimicrobials in animals
As you learned in Section 4.3, there is limited information available on use of antimicrobials in animals. The World Organisation for Animal Health (
In the fourth round of data collection, 152 member states submitted complete reports, of which 118 included quantitative data on animal use. However, based on challenges faced by countries to collect quantitative data on antimicrobial use in animals, the OIE advises caution in interpretation and use of quantitative data. Based on data reported to the OIE from 92 countries, the global estimate of antimicrobial agents used in animals in 2016 adjusted by animal biomass was 144.39 mg/kg (Góchez, 2019; OIE, 2020).
GLASS, coordinated by the WHO
In 2015, the
The Global Antimicrobial Resistance Surveillance System (
Activity 25: The Global Antimicrobial Resistance Surveillance System (GLASS)
Take a look at Figure 10, taken from the 2020 GLASS report (WHO, 2020):
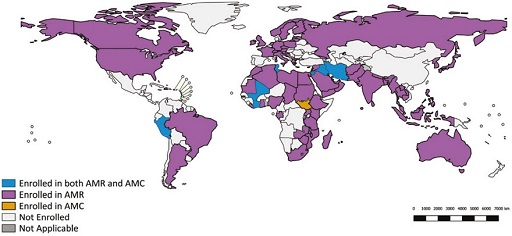
- Is your country enrolled in GLASS?
- If so, is it enrolled for AMR or antimicrobial consumption surveillance, or both?
You will learn more about GLASS in the module An overview of national AMR surveillance.
6 End-of-module quiz
Well done – you have reached the end of this module and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
7 Summary
Bacteria are micro-organisms that can cause diseases in humans, animals and plants. Hygiene measures such as handwashing and water sanitation are important in the prevention of bacterial infections. Antibiotics can be used to treat a bacterial infection. Antibiotics are sometimes referred to as ‘magic bullets’ that kill the infection but keep the patient alive. Antibiotics have played an important role in the treatment of bacterial infections, but also have supported modern human and veterinary medicine in different ways. For example, prophylactic use of antibiotics in surgery reduces the risk of infection. Antibiotics also have a role in food production by keeping plants and food-producing animals healthy.
However, due to resistance that develops in bacteria, antibiotics are becoming less effective in treating bacterial infections. Development of resistance is a naturally occurring process, but due to increased antimicrobial use in human and animal health, we have selected for resistant bacteria. The resistant bacteria can spread directly between humans, between animals and humans, and in some cases via the environment.
The increase in AMR is a serious threat to global health. The global response to fight the problem of AMR includes several activities in the framework of the Global Action Plan on AMR, which uses a One Health approach.
Now that you have completed this module, consider the following questions:
- What is the single most important lesson that you have taken away from this module?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 26: Reflecting on your progress
Do you remember at the beginning of this module you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this module, go to your reflective blog and note down your thoughts.
8 Your experience of this module
Now that you have completed this module, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this module and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Acknowledgements
This free course was collaboratively written by Clare Samson and Dorien Faber, and reviewed by Priya Khanna, Hilary MacQueen, Rachel McMullan,Claire Gordon and Natalie Moyen.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Images
Course image: © Design Cells/iStock/Getty Images Plus.
Figure 1: © Natalie Dee 2002–2018. Courtesy of Natalie Dee.
Figure 2: Center for Disease Dynamics, Economics & Policy (cddep.org). © Natural Earth.
Figures 3–5: taken from https://resistancemap.cddep.org/ AntibioticResistance.php.
Figure 7: from Gelband, H. et al. (2015) The State of the Worlds Antibiotics 2015, https://www.cddep.org/ publications/ state_worlds_antibiotics_2015/. Source: Van Boeckel et al., 2015 (adapted; based on IMS MIDAS).
Figure 8: United Nations Foundation (2017) ‘Sustaining global action on antimicrobial resistance’, Wellcome Trust.
Figure 9: Fleming Fund (2017) ‘What you need to know about antimicrobial resistance’, taken from https://www.flemingfund.org/ wp-content/ uploads/ LP1_AMR_A4Screen_FinalSignOff_Jan2017.pdf.
Figure 10: data, World Health Organization; map, Information Evidence and Research (IER). © WHO 2019.
Videos
Video 1: from Pain, Pus and Poison: The Search for Modern Medicines, episode 2, TX 10 Oct 2013. © BBC.
Video 2: ‘The cure – unsung hero: Ignaz Semmelweis’, 20 August 2013.
Video 3: ‘Seven wonders of the microbe world’, The Open University; Alexander Fleming in his laboratory: © Davies/Stringer/iStock/Getty Images Plus; image from Living Memory: Ena Munroe/The Living Memory Association; image of bacteria-MRSA: Centers for Disease Control and Prevention, USA (public domain); image of Penillium notatum : this file is licensed under the Creative Commons Attribution-Share Alike licence, http://creativecommons.org/ licenses/ by-sa/ 3.0/; image of William Stewart: public domain.
Video 4: BBC Learning Zone. © BBC.
Video 6: TED-Ed; this file is licensed under the Creative Commons Attribution-Noncommercial licence (http://creativecommons.org/ licenses/ by-nc/ 3.0/).
Video 7: Michael Mosley vs The Superbugs, TX 17 May 2017. © BBC/Renegade Pictures.
Video 8: TED, 25 June 2015, https://creativecommons.org/ licenses/ by-nc-nd/ 4.0/.
Video 9: WorldFish; this file is licensed under the Creative Commons Attribution-Noncommercial licence (http://creativecommons.org/ licenses/ by-nc/ 4.0/); text: Global action plan on antimicrobial resistance. https://apps.who.int/ iris/ bitstream/ handle/ 10665/ 193736/ 9789241509763_eng.pdf : World Health Organization; [n.d.]. Licence: CC BY-NC-SA 3.0 IGO.
Video 10: The Association of the British Pharmaceutical Industry, ‘10 steps to reducing AMR – the Review on Antimicrobial Resistance’.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.