Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 25 April 2024, 5:15 AM
Quality assurance and AMR surveillance
Introduction
Microbiology laboratories need to produce test results that are timely, accurate, precise and reliable. Maintaining a high-quality standard in a laboratory is essential in order for doctors, veterinarians, and public health officials to have confidence in the test results the laboratory produces. In antimicrobial resistance (AMR) surveillance, there is the additional challenge of ensuring that data generated in different sectors can be compared or interpreted with reference to other sectors.
In this module, you will learn about the key principles underlying laboratory quality, and the processes a laboratory puts in place to ensure that it operates to a consistently high standard and generates good-quality and reliable data. You will probably be familiar with many of the terms used in the context of quality, and even if you aren’t, many of them may sound similar, for example, ‘quality control’, ‘quality assurance’, and ‘quality management system’.
After completing this module, you will be able to:
- explain the importance of data integrity and quality in laboratory practice in the AMR surveillance context
- outline quality assurance measures in the context of AMR surveillance
- describe the key components of laboratory practice that underpin the quality testing and analysis of antibiotic resistance data
- recognise that your role is an integral part of the AMR surveillance process and that you are responsible for managing quality standards in the workplace
- reflect on the different components of a quality management system and how they are applied in your workplace, and what improvements could be made to ensure that your workplace is obtaining, recording and reporting good quality data.
Activity 1: Assessing your skills and knowledge
Before you begin this module, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Do not worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these modules.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 The importance of data integrity and data quality
It is essential that microbiology laboratories generate data that are fit for purpose (that is, are of high quality) and are trustworthy (have integrity). High-quality data are accurate, consistent, reliable and timely, while data have integrity if they are accurate, validated and complete. You will learn more about these terms in the Fundamentals of data for AMR module.
The principles and practices described in this module minimise the likelihood of an error occurring at each step of the testing process. Doctors and veterinarians must be confident about laboratory reports of test results to inform clinical decisions for a sick person or animal, such as deciding which treatment to give or what additional tests are needed.
For AMR surveillance, pathogen identification and an
Activity 2: Potential harmful consequences arising from data issues
Data quality and integrity are important at each step of the AMR surveillance process. In particular, data issues that arise at a local level can have far-reaching effects. For each of the following situations in this activity we would like you to suggest possible negative outcomes for patient care and/or AMR surveillance.
- The pathogen isolated from an individual specimen is wrongly identified.
Answer
The antibiotic chosen to treat the patient may be inappropriate and the patient’s clinical condition could worsen, possibly leading to death.
- The AST result for an isolate is not interpreted correctly; for example, the inhibition zone diameter is not compared to international standards.
Answer
The patient may receive an ineffective or a sub-optimal dose of antibiotic. Patient care may be compromised.
- The AST methodology is not strictly followed, and different operators use different methods or processes; for example, the McFarland standard is not used.
Answer
Multiple AST results may be inaccurate with the following potential outcomes:
- Individual results are wrongly reported as susceptible/resistant to the chosen antibiotic and patients may receive an ineffective antibiotic or sub-optimal dose.
- The integrity of AST data collected for AMR surveillance is compromised. Policy decision-making by those involved in environmental, public and veterinary health may be affected.
High-quality data generated by microbiology laboratories are the foundation for good patient care and effective AMR surveillance. Incorrect data can negatively impact decision- making at all levels. It is vital that all those employed in a laboratory recognise the need to work to agreed standards of practice and follow measures to ensure that data are reliable.
2 Ensuring quality in the laboratory
What is ‘quality’?
There are many notions associated with the term ‘quality’. For an organisation, quality is associated not only with the products and services it produces to satisfy a demand, but also the processes in place to generate these products or services. In a biomedical laboratory, several factors can affect the final report – the competency of the technician, the laboratory procedure, the reagents used in the assay, the equipment and the facilities. In Figure 1, these and other factors are grouped into 12
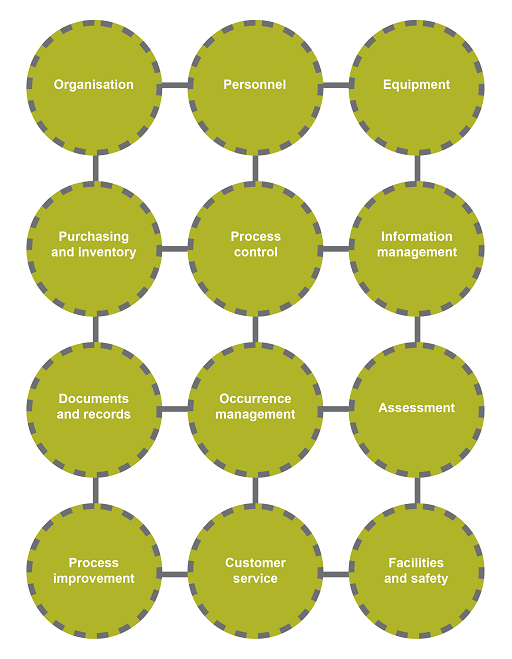
The principles of ensuring high-quality data include:
- making sure that basic standards are met
- establishing procedures at all test stages to minimise errors and ensure results are reproducible and reliable
- measuring performance against international standards
- monitoring and improving practice.
Information must also be recorded and reported accurately. If the laboratory generates a reliable, accurate result but the wrong information is entered into the report, all the hard work done to get the result is wasted.
2.1 Meeting basic standards: facilities and safety
It is important to ensure that as far as possible the laboratory provides a safe and practical working environment.
The size, construction and location of the laboratory facility should be fit for purpose, with separate areas for different activities to optimise working conditions. Staff should have sufficient space for their work, should not have to deal with multiple distractions whilst processing samples, and should have access to the necessary equipment and commodities. Equipment should be in good working order and maintained and serviced as required by the manufacturer. Staff are entitled to expect a clean, safe and secure environment in which to work.
Safety within the laboratory includes:
- general safety – protection from standard workplace hazards such as trips, falls, fires, etc.
- biosafety – the measures taken to protect personnel and the environment from unintentional exposure to potentially infectious agents or to toxins.
Biosafety measures include:
- staff training
- strict adherence to protocols
- wearing personal protective equipment (PPE) such as gloves, lab coat and mask.
When are biosafety practices undertaken in a microbiology laboratory?
All the time.
Something that is often considered alongside biosafety is
Veterinary laboratories must be especially careful to prevent the release of animal pathogens into the wild. Why is this?
Many pathogens isolated in veterinary laboratories not only pose a threat to animal health but they also have the potential to infect humans (in the form of ‘zoonoses’, diseases which can be transmitted to humans from animals).
To ensure staff and public safety, infection control, risk assessments, cleaning and waste disposal should be covered by specific policies. Staff training and the monitoring of best practice should also be carried out with appraisal and feedback. Laboratory manuals should be regularly updated to reflect current biosafety and biosecurity guidance and standards. For clinical laboratories, guidance is provided by the World Health Organization (WHO, 2004, 2006), and for veterinary laboratories, by the manual of diagnostic tests and vaccines for terrestrial animals (OIE, 2019).
Who is responsible for safety and security within the laboratory?
Whatever their role, everyone should (a) be aware of basic safety rules, and (b) be responsible for behaving in a way that does not put themselves or others in danger in the workplace. Risk assessments and staff safety training are the responsibility of those with managerial roles.
Activity 3: Biosafety and biosecurity measures in place in a clinical microbiology laboratory
Look at Figure 2 below.
Identify the elements in the figure that are related to:
- a safe work environment
- biosafety
- biosecurity.
Answer
A safe work environment
- A clear warning sign of caution
- Lab contact phone numbers
- Emergency phone numbers
- A Lab Safety Committee is active in this institution
Biosafety
- A biohazard sign
- A sign instructing no eating, drinking or smoking
- An indication of the biosafety level (BSL2)
- PPE requirement
Biosecurity
- A biohazard sign
- A signpost for a restricted area
2.2 Organisational structure
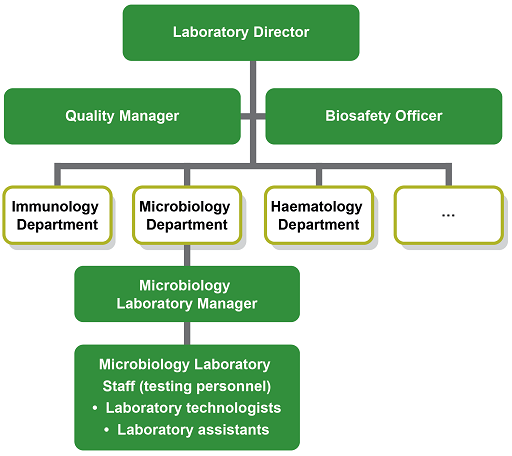
The laboratory should have a clear organisational structure with the roles and managerial responsibilities of all staff members made explicit. The Laboratory Director has overall responsibility and leadership for the running of the laboratory. The Laboratory Manager is responsible for organising the work of laboratory technologists and for maintaining quality standards and safety aspects.
The Quality Manager takes responsibility of the QMS, and for approving all the relevant documentation and processes. Depending on the size of the laboratory, additional roles dealing with biosafety, equipment, supplies, training etc. may be delegated (Figure 3).
(Figure 3 is an example with a focus on the microbiology department of a clinical laboratory. Your workplace may be considerably larger with more sections or considerably smaller with staff members having more than one role. Note that the Quality Manager and Biosafety Officer advise the Laboratory Manager, but do not necessarily directly line-manage staff if resources are scarce or the laboratory is small.)
Activity 4: Your role and responsibility in the workplace (part 1)
Take some time now to think about how your workplace is organised and how your role fits into the organisational structure. You might find it helpful to discuss this with your colleagues using the following questions as a guide:
- Who is responsible for quality policies and standards?
- What responsibilities do you have?
- Do you have more than one role?
- Who is responsible for biosafety?
Discussion
Table 1 summarises the roles and responsibilities regarding the management of quality in a microbiology laboratory.
| Role | Responsibilities |
|---|---|
| Laboratory director |
|
| Quality manager (or safety officer) |
|
| Microbiology staff |
|
2.3 Good microbiological technique
Good microbiological technique refers to the basic technical methods that safeguard human errors and the misuse of equipment responsible for inaccurate results, accidents and laboratory-acquired infections (WHO, 2004). These methods ensure that specimens are transported in an appropriate container and received in a designated area, that samples are opened only by trained staff and handled appropriately when testing, and that samples are stored adequately and disposed of safely. For more about good microbiological practice see the Isolating and identifying bacteria module.
All test samples and cultures should be handled as if they are pathogenic using
Other strategies to avoid cross-contamination of samples and the workplace include careful handling of cultures and keeping the laboratory clean and tidy. Having clearly designated areas for storage of sterile, clean, used/contaminated and used/decontaminated items is recommended.
3 Minimising errors
As noted in Section 1, AMR surveillance relies heavily on local microbiology laboratories producing data of high quality and integrity (Seale et al., 2017). Procedures should be in place to minimise errors, and to make sure that results are reproducible, i.e. the same result will be obtained from a sample even when processed by different operators on different days, or even in different laboratories.
The processes that minimise errors include:
standard operating procedures (SOPs) for all activities- implementing
quality controls (QCs) - ensuring all staff are trained on equipment and procedures.
3.1 Standard operating procedures (SOPs)
SOPs are unambiguous written instructions covering routine laboratory operations as well as the organisation and management of the laboratory. By stipulating how a task should be done, SOPs ensure consistency in the workplace; this in turn reduces testing error and bias and leads to more reliable and accurate results. SOPs should be available for all procedures, and may be developed locally or for a national surveillance system developed nationally with adaptations for the for the local situation if needed.
SOPs use clear language, are easy to follow and have a standard format where the header summarises important information regarding document approval and classification (see Table 2). It is essential that SOPs are reviewed regularly and kept up to date, with previous versions being archived. Staff should be kept informed of updates.
| TLM/MSH Microbiology Department Policy & Procedure Manual | Policy # M1/RESP/11/v05 | Page 1 of 5 |
| Section: Respiratory Tract Culture Manual | Subject Title: SPUTUM (Including Endotracheal Tube and Tracheostomy Specimens) | |
| Issued by: LABORATORY MANAGER | Original date: September 25, 2000 | |
| Approved by: Laboratory Director | Revision date: September 14, 2006 | |
| Annual review date: August 13, 2007 | ||
A well-written, comprehensive SOP should provide all the information needed for a new member of staff to be able to complete the procedure exactly (see Table 3).
| Section | Description (where appropriate) |
|---|---|
| Purpose | |
| Scope | |
| Equipment | |
| Reagents and media | Steps for preparing reagents or refer to other SOPs |
| Supplies | General laboratory supplies/disposables as needed |
| Safety precautions | |
| Sample information/processing | Acceptance/rejection criteria, volume, storage, etc. |
| Quality control(s) | Type, expected values |
| Workflow chart | Optional but can be used as a job-aid |
| Procedure | Step-by-step written instructions |
| Method performance specifications | Analytical sensitivity, method’s limitations |
| Result interpretation | Guidelines to be used |
| Results reporting | Instructions for reporting the handling of critical results |
| References | Useful source of information |
| Appendices (optional) | e.g. a manufacturer’s leaflet |
| Revision history |
The SOP should include the scope, purpose and a step-by-step description of how to perform the entire testing process, including how the results should be analysed and interpreted (CLSI).
Who should develop SOPs and who should approve the contents?
- SOPs should be written by laboratory staff who have a good understanding of the activity to be covered.
- Each SOP should be approved by a quality manager, laboratory manager or similar senior staff member who has the necessary authority.
See Public Health England’s comprehensive catalogue of SOPs for examples.
3.2 Identifying where errors occur
Errors can be introduced at any stage of the path of workflow (as shown in Table 4): before the test takes place (pre-analytical), during the test (analytical) and after the test (post-analytical). It is important to identify where errors can occur and put in place controls and measures to reduce these. Using standardised procedures helps keep errors to a minimum.
| Path of workflow | ||
|---|---|---|
| Pre-analytical | Analytical | Post-analytical |
|
|
|
Footnotes
ID = pathogen species or subspecies identification; AST = antimicrobial susceptibility testingActivity 5: Potential sources of error
Part 1
Table 5 lists some errors that may occur during the testing process in a biomedical laboratory. For each example select the appropriate pathway stage from the drop-down options.
3.3 Quality procedures to identify and minimise errors
Several terms are used to describe different aspects of laboratory quality:
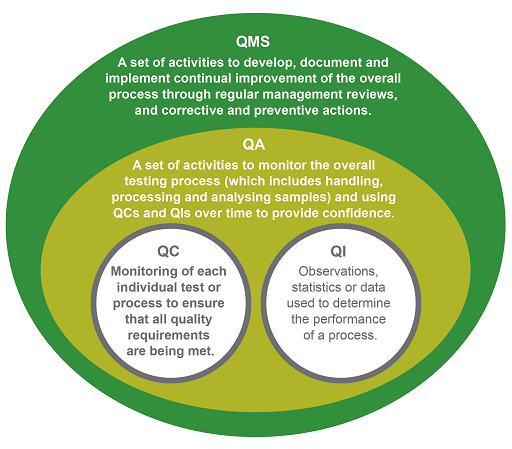
QIs are observations, statistics or data that prove the organisation is meeting its intentions, for example, that its blood culture contamination rate is below 3%. QCs monitor whether the test performs as expected – for example, using a control organism to ensure that reagents or media give the expected result or growth. QA looks at the overall process to confirm that the requirement for quality is fulfilled. This involves tracking QCs and QIs and analysing trends. In the analytical phase, for example, QA ensures that all the relevant SOPs are in place, staff have been trained, the necessary QC checks are being done, and the results are recorded and reported correctly for that process.
Finally, a laboratory QMS is a systematic integrated set of activities to establish and control all processes in the workflow path. The founding principle of a QMS is continual improvement. It manages resources, conducts evaluations and makes continual improvements to ensure consistent quality results. A good QMS ensures that when errors are detected, both preventive and corrective measures are implemented to improve a process (WHO, 2011a; Carey et al., 2018).
3.4 Using controls
A
Microbiology laboratories need to maintain a stock of control organisms, with known characteristics, to provide QC for different tests. For example, when checking the reagents used for an oxidase test, laboratories should use a known oxidase positive organism (usually Pseudomonas aeruginosa) and an oxidase negative organism (such as E. coli). Control strains should be obtained from a reliable source, for example the American Type Culture Collection or the UK National Collection of Type Cultures, and stored and cultured according to the provider’s instruction. Control strains are also used to ensure that selective or indicator media have been prepared correctly.
Activity 6: Using control organisms and quality checks
3.5 Instruments and equipment
As well as looking at processes, a good quality management system (QMS) will make sure that instruments and equipment are functioning correctly. Instruments must be installed and used according to the manufacturer’s instructions. Any new equipment should be validated before being used routinely and kept maintained in full working order, and all staff should be trained on its use.
Equipment and instruments may also need to be calibrated:
Activity 7: Example of a ‘calibration’ SOP
In this activity you will look at an example SOP that covers the calibration of equipment and instruments used in a laboratory.
Read Annex 5 (pp. 39–45) of Laboratory Quality Standards and their Implementation (WHO, 2011b) and answer these questions:
- Whose responsibility is it to train staff to perform calibration/performance checks?
- What factors determine the calibration schedule?
- What action should be taken if an item is found not to be calibrated correctly?
- Is it necessary to recalibrate equipment that has been relocated?
Discussion
- It is the responsibility of the supervisor of the section where the equipment is housed.
- The calibration schedule is determined by the manufacturer’s recommendations, reference standards and recommended time intervals.
- The item should not be used until it is readjusted back into calibration. QC testing may be necessary to assess whether quality has been compromised with measurements taken recently.
- Generally, the item should be recalibrated after relocation, but the manufacturer’s recommendations should be checked.
3.6 Information management
Quality management documents
Examples of laboratory documents which should form part of a QMS are listed in Table 6. All documents must be uniquely identified with revision numbers to avoid the use of outdated versions and made readily accessible to relevant staff member(s).
| Document type | Overview |
|---|---|
| Quality manual | Defines the overarching quality policy and standards. It outlines the organisation, management structure and QMS. |
| Standard operating procedure (SOP) | Provides definitive guidance to carry out a routine activity or process in the laboratory. |
| Job aids | A shortened version of an SOP which briefly describes how to accomplish a task. |
| Biosafety manual | Provides guidance on handling clinical specimens – which should always be treated as potentially hazardous. |
| Technical manual | Provides details of installation, operation, maintenance and training requirements for equipment. |
| Training log | Provides a record of all training undertaken by staff and the completion dates. |
| Records | Electronic or hard copy evidence of results achieved. |
Recording and reporting data
A record is a document stating results achieved or providing evidence of activities performed (WHO 2011a). Records should be complete, legible and carefully maintained. As well as providing information to the prescriber, records allow for continuous monitoring, evaluating problems and informing overall laboratory quality. All the efforts to get the correct result for a sample are wasted if, in the last step, the wrong result is reported to the clinician. Similarly, unless the result is reported in a timely manner it may be of no use to the doctor or veterinarian who requested the test.
For reporting, systems need to be in place to make sure that patient information is correctly recorded and that the final test result is the correct one for that patient. The system may or may not be computerised, but either way, the principles are the same, and every effort should be made to release the report in a clinically relevant timeframe.
Systems (including agreements) should be in place to share data in appropriate formats with patients, doctors/veterinarians/farmers and AMR surveillance systems. Clear guidelines and responsibilities for maintaining and accessing the system are essential, as is staff training.
At all times:
- the accuracy of data recording and transmission should be monitored
- reporting processes should be both effective and timely
- patient and animal-owner confidentiality and privacy should be maintained
- data should be backed-up so that lost data can be retrieved
- the accessibility and security of data should be ensured.
What may happen if laboratory information systems are not robust?
Failure to manage a laboratory’s data effectively can:
- lead to problems with data integrity
- affect several processes which can be used to measure and compare laboratory performance
- affect patient care.
Reputational damage and/or legal repercussions may ensue if the laboratory breaches data protection legislation.
Information management is covered in more detail in the Fundamentals of data for AMR module.
4 Continual improvement
As well as performing all the necessary product and process quality checks, laboratories should be recording, monitoring and measuring their performance to ensure that standards are maintained over time. Laboratories should also strive to identify and promote good practice and look for ways of improving their performance.
4.1 The PDCA model
The
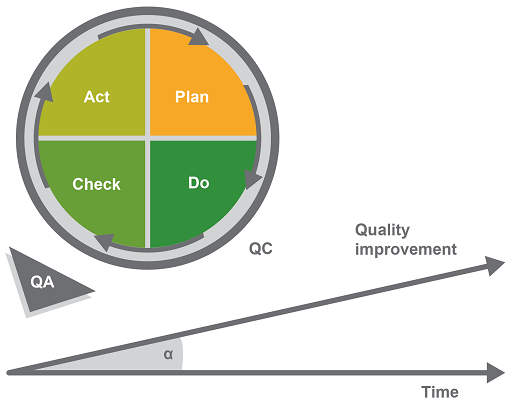
The model has four phases:
- Plan identifies opportunities for improvement and defines an action plan.
- Do corresponds to the pre-analytical, analytical and post-analytical phases of the workflow in a biomedical laboratory. (This may also be called the implementation phase.)
- Check analyses the efficacy of any new measures that have been implemented to see if the process is being improved.
- Act analyses the information from the ‘Do’ and the ‘Check’ phases, validates the approach and sets up corrective and preventive actions, thus improving the overall quality of the QMS.
Preventive and corrective actions
Audits (either internal or external) are formal processes which examine laboratory practices against established standards or regulations. The aim of any audit is to identify areas that are not performing as well as the rest of the laboratory, suggest how to improve turnaround times, and/or streamline workflows so that the laboratory is more efficient and can process more samples. Audits are an excellent method of actively looking for ways to improve a laboratory’s function.
A QMS should identify, log and investigate errors and take measures to address the root cause of a problem. Preventive action addresses problems that have not yet occurred, such as checking a piece of equipment, whereas corrective action is taken to prevent errors from reoccurring. Depending on the severity of the error, actions may range from simply checking a piece of equipment to a more formal investigation which may be necessary if the error has resulted in actual harm. The investigation and measures taken should all be clearly documented.
4.2 External quality assessment
An
If participation in a formal PTS is not possible, assessment of quality may be achieved through a combination of within-country retesting/rechecking by a reference laboratory and internal QA with periodic external observation of practices and procedures by qualified personnel (WHO 2011a). In situations where a PTS for a specific test is not available, interlaboratory comparison to compare results and interpretation can be developed.
4.3 Accreditation
A certificate of
One of the most widely recognised accreditation schemes is the
4.4 Staff training and development
At the most basic level, individuals monitor the quality of their own work. Staff are responsible for making sure that they have been trained on each procedure that they undertake and have read the SOPs and any other relevant documents. Staff are also responsible for making sure that they perform the correct QC checks.
All staff should receive training in health and safety, biosafety and biosecurity, and quality management. Additionally, each member of staff should be trained for the tests that they undertake and in the equipment they use. SOPs should be in place for all staff-related operations, including the recruitment and induction of new personnel, training and assessment.
As well as initial training, ongoing education and training (known as
What training needs might staff have?
Depending on their role, staff could need training in:
- specific job tasks
- quality standards
- internal QCs and QA
- operating equipment
- biosafety
- record-keeping
- computer systems.
All training undertaken should be recorded and the competence of staff assessed regularly with outcomes documented. Each staff member should maintain a training log to document their training progress and help identify any gaps that need to be addressed.
Activity 8: Your role and responsibility in the workplace (part 2)
Take some time now to think about how well prepared and supported you feel in your role. These questions might help you:
- Do you receive regular education and training?
- Would you like to update your knowledge and/or skills? Are there any specific areas you would like training (or further training) in?
- When was the last time your performance at work was assessed?
Discussion
You might find it helpful to talk with colleagues or your Laboratory Manager about these questions.
5 Examples of quality assurance for core processes
Quality assurance should cover every aspect of the test process, from taking the sample to reporting the result. In this section you will consider the QA needed for some core laboratory processes.
5.1 Specimen handling and storage
The accuracy, reliability and timely reporting of test results depend on having good quality samples as a starting point. Laboratories should therefore take proactive steps to ensure that samples received for testing meet their requirements and reject those samples that do not (Figure 7).
Activity 9: Test request form
List the information you would expect to see on a test request form accompanying a sample sent to your workplace.
Discussion
You may wish to compare the two example lists below with the test request form used in your workplace.
Public health
- Patient ID and basic information such as sex, date of birth
- Test requested
- Date and time of sample collection
- Date and time of sample receipt
- Type of sample
- Any clinically relevant information
- Name and signature of the person who authorised the request
- Contact details of person requesting the test
Animal health
- Contact details of owner of animal and/or premises
- Contact details of person requesting the test
- Patient ID and basic information such as gender, age, animal species and breed, type of farm
- Test requested
- Suspected pathogen and tests requested
- Date and time of sample collection
- Date and time of sample receipt
- Type of sample
- Any relevant case history, clinically relevant information
- Any relevant epidemiological information such as spread of infection to other animals, observations about husbandry practices
5.2 Antibiotic susceptibility testing
One of the most important microbiological tests once a bacterial pathogen has been identified is the
In order to achieve accurate and reproducible AST data and comparability of test results between laboratories, using a standardised methodology and test parameters is essential. Adherence to international quality standards such as those set by ISO is a basic requirement. Further standards have been set by the
Laboratories should also ensure that they are using appropriate reference QC strains, and that there is an effective QMS covering all stages of the testing process.
More information on AST can be found in the Antimicrobial susceptibility testing module.
6 Quality assurance in your workplace
You should now have a better understanding of how QA is implemented in microbiology laboratories, and how essential it is to maintaining quality standards. Before you finish this module there are a couple of final activities to complete, which will help you to think about how this knowledge applies to your workplace.
Activity 10: Assessing and improving quality in your workplace
Part 1: Identify resources and regulatory authorities
Guidelines for establishing and/or running a microbiology laboratory may already have been developed in your country. These would include guidance on the essential components of an effective laboratory QMS such as organisation and management, a comprehensive quality manual, and SOPs.
Take a few minutes to research your country-specific guidelines if these are available. You may find it useful to look at reference laboratories, the Ministry of Public Health and the Ministry of Agriculture’s websites.
- Working towards some form of accreditation can provide a framework for improving quality in the laboratory. Many countries have an institution responsible for laboratory accreditation, for example the Medical Laboratory Science Council of Nigeria provides an accreditation service. Take a few minutes to research whether your country has a similar accreditation scheme.
Part 2: Your workplace
- Think about what you have learned in this module and the information you researched in Part 1. Then, on a scale of 1 (low) to 5 (high), rate your workplace for its effective implementation of:
- QC measures
- QA processes
- a QMS
- Are there aspects of how your workplace is run that could be improved? If so, what suggestions would you make?
Discussion
You may find it helpful to discuss your thoughts with colleagues and/or your laboratory manager.
7 End-of-module quiz
Well done – you have reached the end of this module and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
8 Summary
In this module you have learned how a quality management system underpins the operational efficiency of a laboratory, and the internal and external checks that guarantee AMR data quality and integrity. You have also been introduced to the idea of accreditation for laboratories as a way of ensuring that quality standards are maintained.
You should now be able to:
- explain the importance of data integrity and quality in laboratory practice and in the AMR surveillance context
- outline quality assurance measures in the context of AMR surveillance
- describe the key components of laboratory practice that underpin the quality testing and analysis of antibiotic resistance data
- recognise that your role is an integral part of the AMR surveillance process and that you are responsible for managing quality standards in the workplace
- reflect on the different components of a quality management system and how they are applied in your workplace, and what improvements could be made to ensure that your workplace is obtaining, recording and reporting good quality data.
Now that you have completed this module, consider the following questions:
- What is the single most important lesson that you have taken away from this module?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 11: Reflecting on your progress
Do you remember at the beginning of this module you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this module, go to your reflective blog and note down your thoughts.
9 Your experience of this module
Now that you have completed this module, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this module and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Acknowledgements
This free course was collaboratively written by Cyril Buhler and Sarah Palmer, and reviewed by Priya Khanna, Claire Gordon, Natalie Moyen and Hilary MacQueen.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Module image: melpomen/123RF.
Figure 1 and Table 2: reproduced from Laboratory Quality Management System: Handbook, World Health Organization, copyright 2011, https://www.who.int/ ihr/ publications/ lqms_en.pdf (accessed 30 June 2021).
Figure 2: Clinical Microbiology Review, May 2018, 31 (3) e00062-17; DOI: 10.1128/CMR.00062-17.
Figures 3–5: developed by Cyril Buhler.
Figure 6: developed by Cyril Buhler, based on the PDCA cycle by Deming, W. Edwards.
Figure 7: reproduced from template sample rejection form, World Health Organization, copyright 2015, https://extranet.who.int/ lqsi/ content/ template-sample-rejection-form (accessed 26 January 2021).
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.