3.3 Quality procedures to identify and minimise errors
Several terms are used to describe different aspects of laboratory quality:
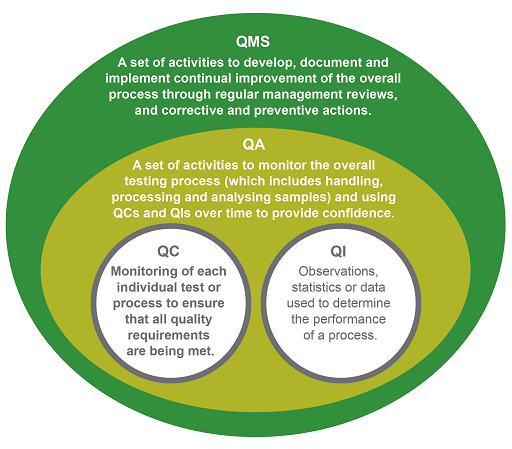
QIs are observations, statistics or data that prove the organisation is meeting its intentions, for example, that its blood culture contamination rate is below 3%. QCs monitor whether the test performs as expected – for example, using a control organism to ensure that reagents or media give the expected result or growth. QA looks at the overall process to confirm that the requirement for quality is fulfilled. This involves tracking QCs and QIs and analysing trends. In the analytical phase, for example, QA ensures that all the relevant SOPs are in place, staff have been trained, the necessary QC checks are being done, and the results are recorded and reported correctly for that process.
Finally, a laboratory QMS is a systematic integrated set of activities to establish and control all processes in the workflow path. The founding principle of a QMS is continual improvement. It manages resources, conducts evaluations and makes continual improvements to ensure consistent quality results. A good QMS ensures that when errors are detected, both preventive and corrective measures are implemented to improve a process (WHO, 2011a; Carey et al., 2018).
3.2 Identifying where errors occur