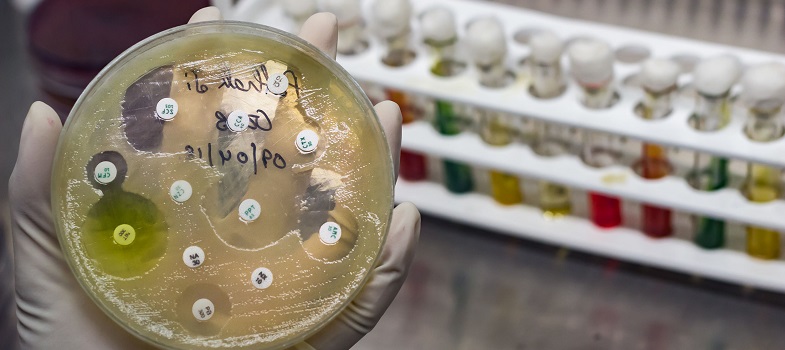
1.2.3 How breakpoints are set
Clinical breakpoints are set collaboratively by a committee of people with a range of backgrounds, including clinical microbiology and infectious diseases.
What factors do you think the committee might have to take into consideration when setting a clinical breakpoint?
It will consider the antibiotic’s mechanism of action and known resistance mechanisms to it; how it is distributed and metabolised in the body – that is, its
pharmacokinetics andpharmacodynamics (PK/PD); and the antibiotic’s MIC distributions and ECOFF values (Toutain et al., 2017; EUCAST, 2019).
Once the committee agrees on an antibiotic breakpoint concentration, this tentative breakpoint is published for consultation and refined before the final breakpoint concentration is agreed. The published guidelines and breakpoints are reviewed regularly, and it is important to always refer to the most up-to-date guidelines available.
Animal and human breakpoints are not necessarily the same, as animals may metabolise some antibiotics differently and so may achieve different tissue concentrations.
1.2.2 Clinical breakpoints