Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 25 April 2024, 4:33 PM
Introducing a One Health approach to AMR
Introduction
This module introduces the concept of One Health and explains the importance of a One Health approach in tackling the AMR crisis. It emphasises how a complex mix of factors involving humans, animals, aquatic species, plants and the environment contribute to the spread of antimicrobial resistance (AMR) within and between sectors.
It introduces the World Health Organization (WHO), the Food and Agriculture Organization (FAO) and the World Organisation for Animal Health (OIE) individually and collectively as the Tripartite, describing their joint role in developing a One Health approach to tackling AMR. The main functions of multi-sectoral organisational structures to address AMR across all sectors in a country will be discussed, with examples.
One Health AMR surveillance is introduced here but will be expanded in the following modules:
- An introduction to AMR surveillance
- Introducing AMR surveillance systems
- An overview of national AMR surveillance
- AMR surveillance in animals.
The main guidelines and protocols that contribute to One Health AMR surveillance will be introduced. As learners you will be encouraged to think about how your work might contribute to a One Health approach to addressing AMR in your country. As noted in other modules, we will focus on bacterial AMR, although the same principles apply to viruses, fungi, protozoa and parasites.
After completing this module, you will be able to:
- explain what is meant by One Health and discuss the importance of this approach in addressing AMR
- describe an example of a One Health AMR problem
- give examples of measures that can be implemented by different sectors in a One Health approach to controlling an AMR problem
- identify the international and national organisational frameworks that support global and national management of AMR
- understand the key features of guidelines and protocols available to support a One Health approach to AMR surveillance
- reflect on how your role fits within a One Health approach to addressing AMR in your country.
We recommend that you have studied The problem of AMR before beginning to work on this module.
Activity 1: Assessing your skills and knowledge
Before you begin this module, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Do not worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these modules.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 What is One Health?
There are many definitions of One Health, but the following definition captures the key aspects:
A collaborative, multisectoral, and trans-disciplinary approach – working at local, regional, national and global levels – to achieve optimal health and well-being outcomes recognizing the interconnections between people, animals, plants and their shared environment
In the context of
- human health and social care
- terrestrial and aquatic animal health and production
- plant health and production
- environmental management.
Professionals from different disciplines include, but are not limited to:
- clinicians
- veterinarians
- pharmacists
- epidemiologists
- microbiologists
- nurses
- sociologists
- data scientists
- economists
- hospital managers
- laboratory technicians
- para-veterinarians.
Collaboration involves sharing information with, and effective communication among, professionals, with the goal of understanding what factors contribute to AMR in each sector, how each sector contributes to AMR in other sectors, and measures for mitigating AMR. Experts from each sector must identify priorities and make decisions together to achieve optimal health outcomes, recognising the interconnections between humans, animals, plants and their environments that contribute to AMR.
One Health not only refers to professionals from different sectors and disciplines working together, but also refers to governments, academic institutions, non-government organisations and community groups working together; for example, to investigate, design and implement appropriate policies and programmes to reduce the use of antimicrobials.
2 Misconceptions about a One Health approach to AMR
Table 1 lists a number of important misconceptions about what is meant by One Health and a One Health approach to AMR. We have provided explanations for why these are not truly One Health approaches to help you understand this module.
| Misconception | Explanation of why these are misconceptions |
|---|---|
‘One Health involves all sectors other than human health. For example, the “One Health sectors” are animal health, environmental health, plant health, etc.’ | This misconception implies that One Health is the responsibility of sectors other than human health. In reality, One Health is the collaboration of all sectors contributing to and affected by AMR – including human health. There is no such thing as a ‘One Health sector’; One Health includes all sectors, especially human health. |
‘The One Health approach is necessary because the overuse of antimicrobials in animals has led to the AMR problem in humans.’ | There is a perception that the use of high volumes of antimicrobials in animals is a major cause of AMR in humans, and that the AMR problem can be mitigated by reducing levels of Scientific evidence does not support the perception that AMU in animals is the major contributor to AMR in humans. While the use of some antimicrobials in animals does contribute to AMR in humans, the current evidence indicates that poor use of antimicrobials in human health care is the major factor driving AMR in humans. |
‘One Health means treating all sectors with the same level of priority.’ | One Health recognises that maintaining the effectiveness of antimicrobials for human health is the highest priority. This may lead to different levels of funding within grants or government budgets in order to address the most important factors associated with AMR in humans. Whilst recognising AMR in humans as a priority, a One Health approach also recognises that AMR impacts the health of animals, aquatic species and plants, and is also important for animal health and welfare, and food security. Hence it is important that the One Health approach respects and understands the AMR problem in all sectors and provides adequate resources to investigate and manage AMR in all affected sectors. |
‘A One Health approach to AMR and AMU surveillance involves implementing the same surveillance methods in all sectors.’ | Approaches to AMR and AMU surveillance may differ between the sectors, depending on the objectives of surveillance in each sector and on the sources of samples from humans, animals, aquatic species and plants. For example, One Health AMR surveillance currently supported by the Fleming Fund is based on the following different surveillance approaches to identify AMR risks for humans in both human and animal populations:
|
‘One Health involves conducting | One Health involves optimising the use of laboratory resources to conduct AST in samples from humans, animals, food and/or the environment. The particular mix of laboratories will depend on each country’s situation. For example, in small countries with a small animal production sector, the optimal use of limited resources may be to test animal samples in the human health laboratory. In countries that do not have laboratories that provide microbiology services for the food and environment sectors, it may be more cost-effective to test food and/or environment samples in animal health or human health laboratories. |
3 The importance of a One Health approach to tackling AMR
AMR is a wicked problem
AMR, together with climate change, is considered to be one of the world’s ‘wicked problems’. Wicked problems are threats to human existence that are very complex to understand, solve or manage. Addressing wicked problems (van Woezik et al., 2016) depends on:
- compromises between competing values
- a multidisciplinary approach
- collaboration between those parties involved .
Wicked problems do not have easy solutions.
A complex interaction of factors drives the emergence and spread of AMR
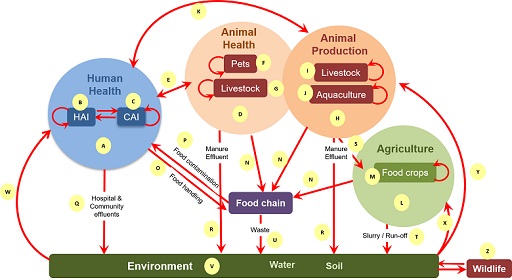
Interaction of an extremely complex mix of factors drives the emergence and spread of AMR within and between populations and their environments, as shown in Figure 1. The figure illustrates the main drivers for emergence of AMR in the different sectors, as well as the transmission pathways within and between sectors.
AMU in humans, animals and plants drives the emergence of resistance within bacterial populations carried by individuals within these populations. Once AMR has emerged it can spread due to poor hygiene, poor waste management or inadequate farm
AMR spread through transmission of resistant bacteria and resistance genes
Bacteria can become resistant to antimicrobials through mutations or through the transmission of
Movement of these elements between bacteria in the host and/or in the environment result in previously non-resistant bacteria becoming resistant. Plasmids are a type of mobile genetic element consisting of circles of DNA that may carry several ARGs. (You were introduced to ARGs and other mobile genetic elements in the Introducing antimicrobial resistance module.) Spread of AMR can therefore be via transmission of the resistant bacteria themselves, or transmission of resistant genes that cause previously susceptible strains to become resistant.
Activity 2: Looking at the complex mix of factors associated with AMR in all sectors
To help understand this complex mix of factors shown in Figure 1, an explanation associated with each letter in the figure is available as a separate PDF file.
Now spend some time exploring Figure 1.
The role of antimicrobial residues in AMR.
Another contributor to AMR are
- excreted by animals and people to whom antimicrobials have been administered and enter rivers from wastewater treatment plants or farm effluent
- present in animal food products if withdrawal periods for antimicrobials used in animals are not adhered to
- enter waterways from pharmaceutical industry waste associated with the manufacturing of antimicrobials.
Antimicrobial residues may contribute to the emergence of AMR in bacteria carried by host populations other than those in which they were initially used. For example, people may consume antimicrobial residues in food derived from treated animals if withdrawal periods are not adhered to, or in water that contains antimicrobial residues from a range of sources (human, agricultural, animal). Exposure to low levels of antimicrobial residues in this way provides a selective pressure that can result in the development of resistance.
In addition, low levels of antimicrobial residues in the environment contribute to the emergence of AMR in naturally occurring environmental bacteria, making the environment a potential reservoir of ARB and ARGs.
AMR management requires a collective effort from all sectors
The management of AMR therefore requires a collective effort from people working with humans, animals, agriculture and the environment to effectively address it.
Activity 3: Reflecting on the complex mix of factors associated with AMR in all sectors
Now that you have explored Figure 1, use the space below to reflect on your own professional experience with AMR. You may find it helpful to refer to Figure 1 and its supplementary PDF file for ideas when you consider sectors you are less familiar with.
- Which sector are you working in? (For example, is it human health, pet animal health, livestock health, aquaculture, wildlife health, plant health, food quality, environmental management or something else?)
- Give an example of an important bacterial AMR problem in the sector that you work in.
- What do you think led to the emergence of this bacterial AMR problem? How is it spreading?
- Can you give an example of an important bacterial AMR problem in another sector?
- Can you describe how AMR in the sector you work in contributes to AMR in populations in another sector?
- Can you describe how AMR in populations in another sector contributes to AMR in populations in the sector you work in?
4 Detection and control of a One Health AMR problem
Let’s look at an example of a One Health AMR problem involving transmission of AMR from animals to humans.
Example of a One Health AMR problem
Transmission of ceftiofur-resistant Salmonella enterica serovar Heidelberg (subsequently referred to as S. Heidelberg) from
You will learn:
- how it was detected through a One Health AMR surveillance system
- what contributed to the resistance emerging in chickens
- how it was transmitted from broilers to people
- a One Health approach to implementing control measures across all sectors.
This example demonstrates that AMR problems involve many factors and cannot be solved by a single approach, or one sector acting in isolation.
4.1 Detection through a One Health AMR surveillance system
The
What does this information suggest about a possible relationship between AMR in chickens and people?
It suggests that ceftiofur resistance in Salmonella in poultry has led to ceftiofur resistance in Salmonella in people, and the resistant bacteria or genes may have been transmitted via the food chain.
The key pieces of evidence for a link between ceftiofur resistance in poultry and people, with transmission through the food chain were as follows:
- There was an increased prevalence of ceftiofur resistance in salmonellae isolated from broilers, chicken meat and people suffering from salmonellosis in the same geographic area during the same time period.
- Serotyping of the Salmonella isolates identified that ceftiofur resistance was occurring in the same Salmonella serovar, i.e. S. Heidelberg, isolated from people, broilers and retail meat.
Further evidence of this transmission of resistance from broilers to people via chicken meat was a drop in prevalence of ceftiofur resistance in S. Heidelberg isolates from infected people and a drop in prevalence in chicken meat following the Quebec poultry industry’s voluntary withdrawal of ceftiofur use in hatcheries that produce broiler chicks, during 2005–6.
Subsequently there was a partial reintroduction of ceftiofur use in hatcheries. Suggest what the result of this might be.
The incidence of resistant organisms might increase. Unfortunately, this is what happened, and resistance increased again in both people and chicken meat.
4.2 Emergence of ceftiofur-resistant S. Heidelberg in broilers
Let’s look at how this ceftiofur resistance emerged in salmonellae in broiler chickens.
The major factor contributing to the emergence of ceftiofur resistance in Salmonella organisms carried by broilers was found to be off-label use of ceftiofur in hatcheries that produce broiler chicks during 2003–4. Small doses of ceftiofur were injected into thousands of eggs immediately prior to hatching and/or into newly hatched chicks to prevent the occurrence of E. coli yolk sac infections (Dutil et al., 2010). This is an example of prophylactic use of antimicrobials to prevent the occurrence of clinical disease and mortality.
At the same time, an increased prevalence of ceftiofur resistance was detected in E. coli as well as in Salmonella isolates from broilers, which was likely to be associated with the same driver: prophylactic use of ceftiofur in broiler hatcheries. This was an additional concern because ceftiofur resistance is carried on a mobile genetic element and can potentially be transmitted from one species of bacteria to another.
4.3 Transmission of AMR from broilers to people
How did the resistance to ceftiofur spread from broilers to people?
Transmission of the ceftiofur-resistant Salmonella organisms from broilers to people occurred via the food chain. People predominantly became infected during preparation and/or consumption of chicken meat carrying the resistant Salmonella organisms. People working on the chicken farms may have been infected through contact with chicken faecal material if they did not follow good personal hygiene practices such as regular handwashing before eating (Collineau et al., 2020).
Figure 2 shows the emergence of ceftiofur-resistant Salmonella organisms in broilers and transmission pathway to people via the food chain.
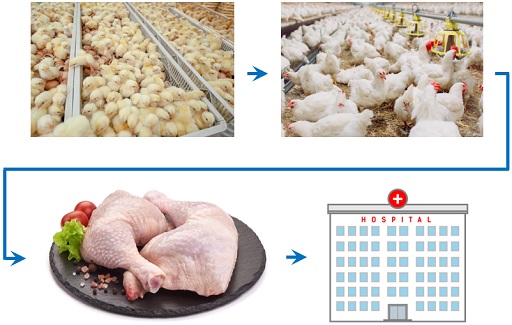
Activity 4: AMR transmission
a.
Not storing chicken meat at sufficiently cold temperatures
b.
Not washing hands after handling raw chicken meat
c.
Poor hygiene when preparing chicken meals, i.e. cross-contamination from uncooked products to cooked products via contaminated bench surfaces or contaminated knives
d.
Undercooking chicken
e.
All of the above
The correct answer is e.
a.
Mixing broilers from infected and non-infected flocks during transport
b.
Transporting broilers in vehicles/crates previously contaminated with chicken faeces.
c.
Using contaminated water and equipment during slaughter and processing
d.
Contact between infected and uninfected broiler carcases and/or chopping boards and knives in retail outlets
e.
All of the above
The correct answer is e.
4.4 The impact on the health of people
Why is ceftiofur resistance in chickens a public health concern?
The public health concern is that use of a
In most countries, ceftiofur is the major 3GC that is used in animals and it is mostly licensed for treating individual sick animals. It is closely related to ceftriaxone, which is the drug of choice for treating severe or invasive salmonellosis in people (Alcaine et al., 2005). There is some evidence of cross-resistance between ceftiofur and ceftriaxone, which may result in 3GC antimicrobials being less effective in treating people with salmonellosis or other serious infections caused by ceftriaxone-resistant E. coli. This can result in people being sick for longer, having a more serious illness and in some cases an increase in mortality. It also leads to the use of alternative antimicrobials such as carbapenems, which are considered a last resort drug for treatment of infections that are resistant to 3GCs.
5 A One Health approach to controlling AMR
Our example AMR problem can be controlled through a One Health approach that involves applying different measures in different sectors at multiple levels that range from global to national to farm to individual people. For example:
- international advocacy and national regulation of 3GC use
- complementary changes in 3GC use at the farm level
- improved hygiene in food processing and retail
- improved hygiene associated with food-handling and preparation in homes
- changes to antimicrobial use in humans.
Let’s think about some of the control measures that can be applied in the different sectors, using the Canadian example of ceftiofur resistance in S. Heidelberg.
Activity 5: Control measures
a.
True
b.
False
The correct answer is a.
Answer
This is the initial step to reduce the emergence of ceftiofur resistance in chicken meat.
a.
True
b.
False
The correct answer is a.
Answer
This will help reduce the spread of Salmonella organisms, including those that are resistant to ceftiofur, among chicken carcases and chicken meat pieces during slaughter and retail.
a.
True
b.
False
The correct answer is a.
Answer
This will help reduce the transmission of Salmonella organisms, including those that are resistant to ceftiofur, from meat to humans during food preparation and consumption.
a.
True
b.
False
The correct answer is b.
Answer
The manufacture of 3GCs will not impact the emergence of resistance through the prophylactic use in hatching eggs and chicks, nor the transmission of ceftiofur resistance from chickens to humans.
In summary, the aim of these measures is to preserve the effectiveness of 3GCs that are important for treating both human and animal diseases by reducing the emergence of resistance, and to reduce the probability of humans becoming infected with Salmonella organisms (resistant and non-resistant) from chicken meat.
There are also measures that can be taken in the environmental sector for controlling salmonellosis in countries with poor water sanitation processes, where water is an important source of infection by Salmonella organisms in addition to the food chain. This expands the illustration of a multi-sectoral approach to controlling transmission of antimicrobial-resistant Salmonellae to include the environment.
5.1 Global-scale measures
OIE guidelines
The
- Not to be used as preventive treatment applied in feed or water in the absence of clinical signs of illness in the animal(s) to be treated.
- Not to be used as first line treatment unless justified. When used as a second line treatment, it should ideally be based on the results of bacteriological tests.
- Extra label/off-label use should be limited and reserved for instances where no alternatives are available. Such use should be in agreement with the national legislation in force.
WHO guidelines
The
The WHO classifies antibiotics for human use into three stewardship groups: Access, Watch and Reserve (AWaRe), as described below. The majority of 3GCs, including ceftriaxone, are in the Watch group, which includes most of the highest priority critically important antimicrobials for human medicine. The WHO recommends that ‘antibiotics in the “Watch” group should be prioritized as key targets of stewardship programs and antimicrobial use monitoring’. The AWaRe database can be accessed from the WHO website (WHO, 2019c).
The WHO’s AWaRe classification

Access antibiotics ‘have activity against a wide range of commonly encountered susceptible pathogens while also showing lower resistance potential than antibiotics in the other groups.’
Watch antibiotics ‘have higher resistance potential and include most of the highest priority agents among the critically important antimicrobials for human medicine and/or antibiotics that are at relatively high risk of selection of bacterial resistance. Antibiotics in the Watch group should be prioritized as key targets of stewardship programs and monitoring.’
Reserve antibiotics ‘include antibiotics and antibiotic classes that should be reserved for treatment of confirmed or suspected infections due to multi-drug-resistant organisms. Antibiotics in the Reserve group should be treated as “last resort” options.’
5.2 National-scale measures: industry controls and national regulations
The following measures have been taken in a range of countries to reduce ceftiofur resistance in salmonellae carried by chickens:
- The poultry industry in multiple Canadian provinces voluntarily withdrew the prophylactic use of ceftiofur in hatching eggs and chicks during the early 2000s (Dutil et al., 2010).
- In May 2014, the Canadian chicken industry voluntarily withdrew the preventive use of all Category I antimicrobials, i.e. those considered by WHO as the most critically important to human health, which included ceftiofur (Collineau et al., 2020).
- In Japan, the poultry industry ceased using ceftiofur in 2012, following which the prevalence of 3GC-resistant Salmonella in chicken meat significantly decreased (Collineau et al., 2020).
- Denmark implemented voluntary restrictions on ceftiofur use (Collineau et al., 2020).
- Europe removed the ceftiofur label claim for injection of day-old chicks (Collineau et al., 2020)
- The US banned off-label use of ceftiofur in hatcheries (Collineau et al., 2020).
5.3 Farm-level measures
Improvements in biosecurity, hygiene and incubation conditions on broiler breeder farms can replace the need for prophylactic use of antibiotics by reducing the risk of E. coli infection in newly hatched chicks. These measures reduce the risk of infections being introduced to the farm and further spread within the farm environment (Collineau et al., 2020). For example, chickens can be infected with Salmonella organisms from their environment. Possible sources are contaminated feed, rodents or contact (direct or indirect) between infected and uninfected birds.
Hygiene is vital in all areas, and education and support for poultry farmers is therefore key.
5.4 Food processing and retail

Cross-contamination resulting in the further spread of Salmonella organisms can occur when chickens are transported in a vehicle previously used to transport chickens carrying ceftiofur-resistant salmonellae. Cross-contamination can occur at the chilling stage of processing chicken carcasses, for example if water used for chilling becomes contaminated.
If there is any residual contamination with Salmonella organisms, further growth of the bacteria is prevented by maintaining the temperature below 4°C at all steps up to and including retail (Collineau et al., 2020).
5.5 Household measures
Improved food-handling hygiene by the consumer, maintaining meat at temperatures below 4°C following purchase, and correct cooking practices will reduce the incidence of salmonellosis in people (Collineau et al., 2020). General good hygiene in the household will also prevent person-to-person spread if an individual does become infected.
5.6 Use of antimicrobials in humans
Modelling the data from the Canadian example suggests that if humans have recently taken antimicrobials before exposure to S. Heidelberg, they are at increased risk of infection with this serovar. This is because antimicrobials in general reduce the bacterial diversity in the human intestine, killing microbes that would normally compete with the pathogenic bacteria.
Therefore, exposure of an individual to resistant bacteria, while they are taking an antibiotic for a different reason, will enable colonisation with that resistant strain (Collineau et al., 2020). A general focus on human antibiotic stewardship and the reduction of use is therefore likely to reduce infection with 3GC-resistant S. Heidelberg, and in principle with any resistant food-borne bacteria.
5.7 Controlling water-borne transmission of AMR
In countries with poor water sanitation systems, water is an important pathway for transmission of antimicrobial-resistant Salmonella organisms in addition to the food chain and direct transmission (Afema et al., 2016).
In these countries, the environment sector can contribute to controlling the spread of Salmonella organisms by improving the management of waterways to reduce the contamination of freshwater with untreated sewage.
6 Leading the fight against AMR at the global level: the Tripartite
The
- World Health Organization (WHO)
Food and Agriculture Organization (FAO) - World Organisation for Animal Health (OIE), formerly the Office International des Epizooties (OIE).
They have signed a Memorandum of Understanding to work together to lead a collective global One Health approach that minimises the emergence and spread of zoonotic diseases and AMR (OIE et al., 2018).
The Tripartite has developed a draft workplan to combat AMR (OIE, 2019c) that aims to:
- ensure that antimicrobial agents continue to be effective and useful to cure diseases in humans and animals
- promote prudent and responsible use of antimicrobial agents
- ensure global access to medicines of good quality.
Activity 6: Looking at the Tripartite
Part 1
Spend a few minutes exploring the separate roles of the three organisations in relation to AMR, then answer the questions in Part 2.
- Visit the WHO’s AMR website (WHO, n.d. 1) and scroll down to the section labelled ‘Our work’. The WHO states that its core role is to ‘co-ordinate the global AMR response in collaboration with key partners’.
Visit the AMR section of the OIE website (OIE, n.d.) and spend a few minutes browsing the information.
Key roles of OIE include the following:
- Guiding the prudent use of antimicrobials in terrestrial and aquatic animals.
- Developing intergovernmental standards on AMR and on monitoring the quantities of antimicrobial agents used.
- Establishing a worldwide database to monitor the use of antimicrobial agents in animals. The OIE supports member countries to develop systems for measuring and reporting antimicrobial use in animals at the country level. The purpose of this system is to improve countries’ capability to reliably measure AMU in different animal production systems, which will contribute to a global and regional analysis of antibiotic use in animals and indicate changes over time.
Visit the AMR section of the FAO website (FAO, n.d.) and look at The FAO Action Plan on Antimicrobial Resistance 2016–2020 (FAO, 2016).
Key roles of FAO include the following:
- Supporting governments, producers, traders and other stakeholders to move towards the responsible use of antimicrobials in agriculture.
- Setting international standards for food and animal feed, through its role of hosting the Codex Alimentarius, or ‘Food Code’: a collection of standards, guidelines and codes of practice adopted by the Codex Alimentarius Commission. The Commission, also known as the CAC, is the central part of the Joint FAO/WHO Food Standards Programme and was established by the FAO and the WHO to protect consumer health and promote fair practices in food trade.
- Supporting AMR surveillance in animals and food by producing the following resources: Regional Antimicrobial Resistance Monitoring and Surveillance Guidelines: Volume 1, Monitoring and Surveillance of Antimicrobial Resistant Bacteria from Healthy Food Animals Intended for Consumption (FAO, 2019) and the FAO Assessment Tool for Laboratories and AMR Surveillance (FAO-ATLASS) (FAO, n.d.).
a.
FAO
b.
OIE
c.
WHO
The correct answer is c.
Answer
The WHO Integrated Antimicrobial Stewardship toolkit provides practical guidance on setting up antimicrobial stewardship (AMS) programmes in the human health sector (WHO, 2019b).
a.
FAO
b.
OIE
c.
WHO
The correct answer is b.
Answer
The OIE provides a list of antimicrobial agents recommended for use in food-producing animals, separating these into levels of importance with a view to preserving their efficacy.
a.
FAO
b.
OIE
c.
WHO
The correct answer is a.
Answer
For example, the FAO hosts the Codex Alimentarius, which provides guidelines designed to minimise the transmission of AMR through the food chain. You can read about Codex Alimentarius on the FAO website.
a.
FAO
b.
OIE
c.
WHO
The correct answer is c.
Answer
This is called the Global Antimicrobial Resistance Surveillance System (GLASS), which you can read about on the WHO’s GLASS website. There is also more information in the Introducing AMR surveillance systems module.
a.
FAO
b.
OIE
c.
WHO
The correct answer is a.
Answer
For example, the FAO’s own action plan on AMR aims to increase existing laboratory capacities for monitoring AMR and detecting antimicrobial residues (FAO, 2016).
a.
FAO
b.
OIE
c.
WHO
The correct answer is b.
Answer
The OIE supports surveillance of AMU in animals by providing a system and tools for countries to report AMU in animals at the country level (OIE, 2020).
Discussion
You may have found it difficult to identify the relevant organisation for each activity because there is some cross-over, so don’t worry. This is one reason why it is so important that these organisations collaborate!
You probably noticed that all the organisations mention the importance of a One Health approach, and are committed to implementing the Global Action Plan for AMR (WHO, 2015), which has five strategic objectives to:
- improve awareness and understanding of AMR
- strengthen knowledge through surveillance and research
- reduce the incidence of infection
- optimise the use of antimicrobial agents
- develop the economic case for sustainable investment that takes account of the needs of all countries, and increases investment in new medicines, diagnostic tools, vaccines and other interventions.
7 National multi-sectoral organisations that address AMR
There is a range of multi-sectoral organisational structures across different countries to address the major functions needed for a One Health approach to control AMR. The five major functions are described in Table 2 with a description and examples of the name of national organisations addressing each function.
| Function | Description | Example |
|---|---|---|
Policy and budgetary allocation | Ministerial-level collaboration that endorses AMR policies in each sector and agrees on budgetary allocation. Some countries have a designated organisation to support policy-making related to One Health programmes (which includes AMR), while in others the senior government members of the national AMR committee may agree on policy. In other countries, AMR policy may be endorsed through other government mechanisms. | Inter-Ministerial Committee for One Health (IMCOH), Bhutan |
National leadership of the AMR programme | A multi-sectoral committee responsible for strategic planning and oversight of the National Action Plan for AMR, e.g. National AMR Coordination Committee (AMRCC). | Multi-sectoral Coordinating Committee (MCC), Tanzania Antimicrobial Resistance Multisectoral Steering Committee (AMRMSC), Nepal |
Stakeholder engagement | Some countries have a designated platform for engaging with the broad range of AMR stakeholders, which may include government, academic, non-government, professional, community and industry organisations. This platform functions to support awareness of AMR and the design and implementation of programmes to mitigate AMR. | One Health AMR platform, Uganda |
AMR technical leadership | One Health Technical Working Committees (TWCs) or Technical Working Groups (TWGs) that comprise experts from AMR-related disciplines from each sector provide multi-sectoral technical leadership for AMR management and advise the AMRCC on policies and programmes. In some countries the TWC or TWG is a sub-committee of the national AMRCC. | National Technical Working Committee (NTWC), Nepal |
National drug administration | Organisation(s) responsible for the national administration of drug importation and overseeing the responsible use of antimicrobials to preserve their effectiveness to treat human, animal and plant diseases. In many countries a single authority administers drugs for both humans and animals, while in others, separate organisations administer drug importation and use in human and animals. | National Drug Authority, Uganda National Drug Technical Advisory Committee, Bhutan |
Activity 7: Multi-sectoral organisations that address AMR in your country
Look again at Table 2 use the space below to note down the names of the national organisation(s) in your country that address each of these functions.
8 One Health AMR surveillance programmes
You will learn more about bacterial AMR surveillance in the following modules:
- An introduction to AMR surveillance
- Introducing AMR surveillance systems
- An overview of national AMR surveillance
- AMR surveillance in animals.
Here we will just introduce the concept of a One Health approach to AMR surveillance.
What is One Health AMR surveillance?
One Health AMR surveillance:
- is a multi-sectoral surveillance programme to detect and monitor trends in ARB and/or ARG that contribute to AMR in multiple sectors
- generally focuses on measuring resistance to a common set of antimicrobials in a common set of
zoonotic bacteria in humans, animals and/or food products and environmental compartments such as water or soil - helps to identify ARB and/or ARG in humans that have spread from animals and the pathways by which they have been transmitted
- may focus on investigating the role of the environment in transmitting ARB and ARG that occur only in humans or only in animals.
In addition to this specific focus on ARB and/or ARG that may contribute to resistance in multiple sectors, multi-sectoral sharing of each sector’s broader AMR surveillance results helps the multi-sectoral AMR Technical Working Group (TWG) and the AMR Coordinating Committee (AMRCC) understand the multi-sectoral epidemiology of AMR in their country. This broader understanding is important for building a multi-sectoral approach to managing AMR.
Figure 3 shows an example of a One Health AMR surveillance programme involving the human health and animal health sectors, with each sector sharing and interpreting their combined surveillance results, using them to update the AMRCC with information generated through surveillance, and recommendations for policy and programmes. The programme can include other sectors such as the environment and/or food quality management as these sectors become engaged.
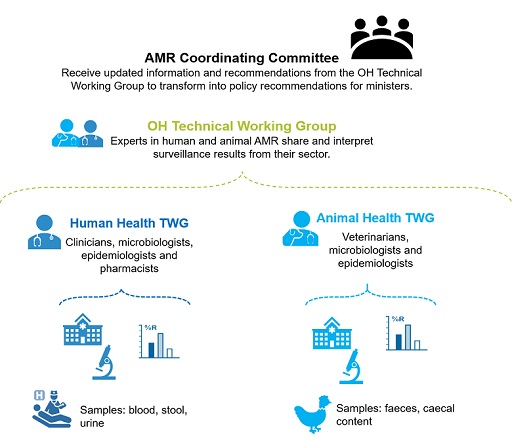
The aims of One Health AMR surveillance are to:
- identify the ARB and/or ARG that are transmitted between humans, animals, aquatic species and/or plants
- contribute to an understanding of pathways by which the ARB and/or ARG are transmitted between populations in the different sectors
- provide data for assessments of the public health risk of zoonotic ARB and/or ARG that are transmitted to humans and the effect of possible intervention measures
- monitor trends in ARB and/or ARG that are transmitted between populations in the different sectors.
- evaluate interventions aimed at reducing the emergence and/or transmission of ARB and/or ARG within and between populations.
It should be noted that One Health AMR surveillance should be conducted in conjunction with One Health AMU surveillance so that information on AMU is used to inform the design of AMR surveillance, and feeds into the interpretation of AMR data.
There are examples of effective One Health AMR surveillance programmes in a number of countries, including the Canadian CIPARS programme described in Section 4.1 that was responsible for detecting the transmission of ceftiofur-resistant S. Heidelberg from broiler chickens to people via the food chain. CIPARS monitors trends in AMU and AMR in selected bacterial organisms from human, animal and food sources across Canada. (More information can be found on the Canadian government’s website (Government of Canada, 2006).)
As yet there are no examples of effective One Health Bacterial AMR surveillance systems in low- and middle-income countries, which is a major reason for the Fleming Fund programme supporting their development and implementation in a range of countries.
What makes a One Health AMR surveillance system ‘One Healthy’?
We suggest the following points:
- Surveillance includes AST of a common set of antimicrobials, using a common set of standards, in a common set of bacteria in samples from multiple sectors, e.g. from humans, animals and/or food and water from the environment.
- Common AMR patterns in samples from the different sectors are further investigated (e.g. using molecular and epidemiological methods) to provide evidence of transmission of resistant bacteria between the different sectors.
Activity 8: AMR surveillance and you
How does your role contribute to AMR surveillance? Do you meet with colleagues from other sectors to discuss AMR surveillance results?
9 Guidelines, standards and protocols for One Health AMR and AMU surveillance
The WHO and FAO guidelines are summarised in this section, as well as the OIE’s set of standards to support One Health AMR surveillance that aims to identify public health AMR risks associated with bacteria carried by healthy animals raised for human consumption. Also included is the AMR surveillance protocol that Mott MacDonald produced to support countries design an active surveillance programme for AMR in healthy broiler and layer poultry. There’s also a brief overview and key features of the document produced by each organisation.
As described in Section 8, One Health AMR surveillance measures resistance to a common set of antimicrobials in a common set of zoonotic bacteria in samples from humans, animals and/or food products and the environmental. All of the documents support a One Health approach to AMR surveillance by focusing on measuring resistance in zoonotic bacteria to antimicrobials that are of critical importance to human health.
The WHO guidelines guide integrated surveillance in the common set of bacteria and antimicrobials in samples from humans, animals and food products. While the other three documents do not guide surveillance in humans, they support the animal health sectors to design and implement their contribution to One Health AMR surveillance through testing samples from animals and/or animal-derived food products. An important factor of One Health AMR surveillance is to harmonise AST methods and interpretation of results across samples from humans, animals and/or food products.
9.1 WHO
 | WHO-led Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR), in collaboration with FAO and OIE (WHO, 2017) |
Guidelines for the integrated surveillance of AMR in food-borne bacteria from humans, animals and food.
- Supports a One Health approach to AMR surveillance by targeting AMR in zoonotic bacteria that may spread from animals to people, focusing on antimicrobials that are critically important for human health.
- Focuses on integrating AMR surveillance in humans, animals and food.
- Presents harmonised methods for AST and criteria for interpreting results in bacteria from humans, animals and food.
- Presents issues to consider when analysing and reporting AMR surveillance results.
- Extensive section on surveillance for AMU in humans and animals, including AMU in hospitals, communities, on farms and sales of antimicrobials for use in animals.
- Provides examples of integrating and graphically comparing AMR and AMU data from people, animals and animal products.
9.2 FAO
Regional guidelines for AMR surveillance in zoonotic bacteria carried by healthy animals raised for human consumption and animal-derived food products focused on south-east Asia.
- Supports a One Health approach to AMR surveillance by targeting AMR in zoonotic bacteria that may spread from animals to people, focusing on antimicrobials that are critically important for human health.
- Focuses on AMR surveillance in food-producing animals and food products.
- Emphasises the importance of representative sampling to generate an unbiased estimate of AMR prevalence in target bacteria.
- Comprehensive description of laboratory methods and guides AMR data management, analysis and interpretation.
- Presents AMR surveillance planning tools and a regional template for AMR data collection.
- Does not cover AMU surveillance.
- Can readily be adapted and contextualised for AMR surveillance in other regions.
- First of a set of five AMR and AMU surveillance guidelines that the FAO is in the process of producing. Additional guidelines will address AMR surveillance in clinically ill animals and in aquaculture, as well as antimicrobial use at the farm level.
9.3 OIE
 | ‘Harmonisation of national antimicrobial resistance surveillance and monitoring programmes’ OIE (2019a), with an equivalent set of standards for AMR and AMU surveillance in aquatic species in Chapters 6.4 and 6.3, respectively, of the Aquatic Animal Health Code (OIE, 2019b) |
OIE standards for AMR and AMU surveillance in terrestrial and aquatic animals raised for human consumption and in animal-derived food products.
- Supports a One Health approach to AMR surveillance by targeting resistance to priority antibiotics for human health in zoonotic bacteria that may be transmitted to humans.
- Focuses on AMR surveillance in animals, animal-derived food products and animal feed.
- Strong focus on representative sampling of populations to generate unbiased estimates of AMR prevalence in zoonotic bacteria.
- Discusses sample sources, sample types and associated surveillance outputs.
- The AMU surveillance guidelines describe sources of AMU data, types of AMU data and reporting formats, with a short section on interpreting results.
9.4 Mott MacDonald
 | Mott MacDonald, Management Agent for the Fleming Fund Programme |
Guides animal health officials in the countries supported by the Fleming Fund Country Grants (FFCG) programme to design and implement an active surveillance programme for AMR in zoonotic bacteria carried by healthy broilers and chickens.
- Aligned with the guidelines prepared by the FAO, OIE and AGISAR and helps countries to implement these broader guidelines within the framework of the FFCG programme.
- Supports a One Health approach to AMR surveillance by targeting resistance in zoonotic bacteria, aligning with the bacteria targeted in the above guidelines, focusing on antimicrobials that are critically important for human health.
- Focuses on AMR surveillance in healthy poultry.
- Presents options that enable countries to adapt and contextualise the surveillance based on their poultry production and marketing systems, laboratory capacity, and existing AMR surveillance capability and information.
- Provides guidance for selecting surveillance areas and for designing a representative sampling plan that will generate unbiased estimates of AMR prevalence in the target bacteria.
- Does not guide AMU surveillance.
9.5 Reflecting on the One Health guidelines
Now try Activity 9.
Activity 9: Reflecting on the One Health guidelines
a.
They guide surveillance for AMR in bacteria carried by humans.
b.
They guide surveillance for AMR in pathogens that cause clinical disease in animals.
c.
They guide surveillance for resistance to all antimicrobials in all bacteria.
d.
They guide surveillance for AMR in bacteria that may be transmitted from animals to humans.
The correct answer is d.
Discussion
One Health AMR surveillance focuses on surveillance for AMR in zoonotic bacteria carried by animals and the same bacteria carried by people. Zoonotic bacteria may be spread from animals to humans via direct contact with animals, via animal-derived food products or via the environment.
a.
Antimicrobials that are commonly used in human health.
b.
Antimicrobials that are critically important for human health.
c.
Antimicrobials that are critically important for animal health.
d.
Antimicrobials that are commonly used in animal health.
The correct answer is b.
Discussion
One Health AMR surveillance focuses on testing for resistance to antimicrobials that are critically important for human health, in zoonotic bacteria carried by animals and the same bacteria in humans. Critically important antimicrobials for human health are those that are the only available antimicrobial, or one of limited available therapies, to treat serious bacterial infections in people.
a.
Regional Guidelines for Monitoring and Surveillance of Antimicrobial Resistant Bacteria from Healthy Food Animals Intended for Consumption (FAO)
b.
A Protocol for Active AMR Surveillance in Poultry (Mott MacDonald)
c.
‘Harmonisation of national antimicrobial resistance surveillance and monitoring programmes’ (OIE)
d.
Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach (WHO)
The correct answer is d.
Discussion
All protocols focus on surveillance for resistance to critically important human antimicrobials in zoonotic bacteria. WHO’s document provides guidance for implementing surveillance in humans as well as animals and food. The FAO, OIE and Mott MacDonald documents provide guidance for implementing surveillance in animals and food, supporting these sectors to contribute to a One Health AMR surveillance programme.
10 End-of-module quiz
Well done – you have reached the end of this module and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
11 Summary
In this module you learned that AMR is an extremely complex health problem involving interconnections between humans, animals, plants and their environments, and it cannot be effectively addressed by each sector working independently. You learned the definition of One Health and worked through a real-world example of how a One Health approach was applied to detect and reduce the transmission of AMR from animals to humans via the food chain. You should now be able to give examples of measures for controlling a One Health AMR problem that can be implemented at global, national, food processing and retail, farm, household, and individual levels. You had a brief introduction to One Health AMR surveillance and should be able to recognise guidelines, standards or protocols that can assist you to design and implement a One Health AMR surveillance system.
You learned that the One Health approach to addressing AMR is led at the global level by the Tripartite, comprising the WHO, the FAO and the OIE. You also learned about the roles of a range of national multi-sectoral organisations for controlling AMR. You should be able to name the multi-sectoral organisations that are addressing AMR in your country and identify if and/or how your work contributes to a One Health approach to addressing AMR.
You should now be able to:
- explain what is meant by One Health and discuss the importance of this approach in addressing AMR
- describe an example of a One Health AMR problem
- give examples of measures that can be implemented by different sectors in a One Health approach to controlling an AMR problem
- identify the international and national organisational frameworks that support global and national management of AMR
- understand the key features of guidelines and protocols available to support a One Health approach to AMR surveillance
- reflect on how your role fits within a One Health approach to addressing AMR in your country.
Now that you have completed this module, consider the following questions:
- What is the single most important lesson that you have taken away from this module?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 10: Reflecting on your progress
Do you remember at the beginning of this module you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this module, go to your reflective blog and note down your thoughts.
12 Your experience of this module
Now that you have completed this module, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this module and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Acknowledgements
This free course was collaboratively written by Joanna McKenzie and Dawn Harmon, and reviewed by Priya Khanna, Claire Gordon, Natalie Moyen and Hilary MacQueen.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Module image: © Sudowoodo/iStock/Getty Images Plus.
Figures 1 and 3: Mott McDonald.
Figure 2: hatching broiler chicks, © chayakorn lotongkum/iStock/Getty Images Plus; broiler chicken farm, © davit85/iStock/Getty Images Plus; chicken being prepared in a household, GSDesign; hospital, Alexey Hulsov/Pixabay.
Section 5.1: © junpinzon/iStock/Getty Images Plus.
Section 5.4: © Flatfeet/Shutterstock.
Section 5.5: RitaE/Pixabay.
Section 5.6: Ulrike Leone/Pixabay.
Section 5.7: © Weerayuth Kanchanacharoen/123RF.
Section 9.1: Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
Section 9.2: Food and Agriculture Organization of the United Nations, 2019, Monitoring and surveillance of antimicrobial resistance in bacteria from healthy food animals intended for consumption, http://www.fao.org/ 3/ ca6897en/ CA6897EN.pdf. Reproduced with permission. Image: © FAO/Jim Caro.
Section 9.3: World Organisation for Animal Health.
Section 9.4: Fleming Fund.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.