Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Wednesday, 8 May 2024, 6:00 PM
Antimicrobial resistance in animals
Introduction
Welcome to Antimicrobial resistance in animals. This module is aimed at animal health professionals, including laboratory scientists, veterinary services professionals, policy-makers and data scientists such as epidemiologists.
This module builds on information provided in the introductory modules, exploring in depth the challenge of antimicrobial resistance (AMR) in animals. We will only consider bacterial resistance in this module rather than, for example, resistance in parasitic worms. The module begins by covering how and why antimicrobials are used in animals, and how this influences the emergence of resistance in bacteria within animals. Then you will explore pathways for transmission of resistance between animals, humans and the environment. Finally, you consider how different types of data can be used to address the AMR challenge. There are opportunities to reflect on how the principles from the module can be applied in your own setting.
Antimicrobials are compounds that kill or inhibit the growth of any type of microorganism. In this module, we will use the term ‘antimicrobial’ to refer to any drug that is active against bacteria, i.e. antibacterial medicines. We include poultry when using the term ‘livestock’, and ‘food-producing animals’ refers to livestock and aquaculture species. We use the term ‘
After completing this module, you will be able to:
- describe the ways in which antimicrobials are used in animals
- describe the main mechanisms by which AMR in animal production systems may influence the occurrence of resistance in human pathogens, and other routes influencing the occurrence of resistant bacteria in animals and the environment
- explain the consequences of resistant bacteria in animals for animal health, food production and human health
- explain why monitoring AMR in food animal systems (including samples from healthy animals) is critical for tackling the AMR crisis
- illustrate the links between animal health, human health and the environment in animal production systems in relation to AMR
- apply scientific terminology related to AMR when explaining your current work.
Activity 1: Assessing your skills and knowledge
Before you begin this module, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Do not worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these modules.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 The use of antimicrobials in animals
In this section, we explain the different types of
After completing this section you will be able to:
- describe the ways in which antimicrobials are used in animals
- apply scientific terminology related to AMR when explaining your current work.
1.1 Antimicrobials in food production animals
Both livestock and aquatic food production systems have intensified in the last few decades. In low- and middle-income countries (LMICs),
These systems have relied on the use of antimicrobials to ensure animal health and maintain productivity and profitability. In some cases, antimicrobials have been used as substitutes for good management practices that prevent disease, such as strict hygiene and biosecurity. The use of antimicrobials in animal production is an important driver of the emergence of resistant bacteria in animals, which can then spread to humans and the environment.
In 2015, it was estimated that in the US, 70% of medically important antimicrobials are used in agriculture (O’Neill, 2015). Despite reduction efforts, current global projections of
1.2 The role of antimicrobials and different types of use
Antimicrobials play an essential role in promoting animal health and welfare, and therefore are crucial to food security. Antimicrobials can be administered to animals orally in feed or water, or non-orally, for example by injection or via the intra-mammary route.
Antimicrobials may be used in various ways for food-producing animals:
Therapeutic use : Only animals presenting signs of disease are treated. This is the most appropriate use of antimicrobials.Metaphylactic use : Antimicrobials are administered to healthy animals belonging to the same group as animals with clinical signs. This pattern of treatment can occur in poultry and aquaculture production, when antimicrobials are administered in water or feed even though signs are observed only in a few animals. Although therapeutic use is the most appropriate approach, in these systems it is not practical to treat individual animals.Prophylactic use : Antimicrobials are administered to a herd or flock of animals at risk of a disease outbreak but are not currently showing signs of disease.
Antimicrobials have also been used for
Consider the following four examples of AMU and decide whether they are therapeutic use, prophylactic use, metaphylactic use or growth promotion.
A farmer gives three unwell pigs an injection of an antimicrobial.
a.
Therapeutic use
b.
Prophylactic use
c.
Metaphylactic use
d.
Growth promotion
The correct answer is a.
A broiler unit uses low doses of an antimicrobial mixed into the feed to increase productivity.
a.
Therapeutic use
b.
Prophylactic use
c.
Metaphylactic use
d.
Growth promotion
The correct answer is d.
A fish farmer adds antimicrobials to the feed of their fish after a neighbouring fish farm has an outbreak of disease.
a.
Therapeutic use
b.
Prophylactic use
c.
Metaphylactic use
d.
Growth promotion
The correct answer is b.
A dairy farmer treats all their healthy lactating cows with an antimicrobial after four of the cows show signs of mastitis.
a.
Therapeutic use
b.
Prophylactic use
c.
Metaphylactic use
d.
Growth promotion
The correct answer is c.
There are
Examples of factors that may change the risk of AMR development and transmission include:
- unnecessary or inappropriate use, such as using the wrong antimicrobial or treating animals that do not need to be treated
- non-therapeutic use; that is, prophylactic and metaphylactic use, or growth promotion
- the route of administration and formulation, or oral administration versus non-oral administration
- the
bioavailability of the antimicrobials, which determines the amount absorbed versus the amount excreted – if a drug has low bioavailability, more of the antimicrobial will be excreted in the faeces.
Using antimicrobials appropriately, as discussed in the Antimicrobial stewardship in animal health module, can reduce the risk of AMR emergence.
1.3. The importance of regulating AMU in animals
The benefits of regulating antimicrobials in animals have been demonstrated using data from surveillance systems. For example, studies have shown a correlation between the quantity of antimicrobials used in animals and the development of AMR in bacteria in these animals. Several studies in EU countries have shown that policies to reduce
Many antimicrobials used in food-producing animals contain the same active ingredients as those used in human medicine, even though the trade names may differ. The WHO has developed a list of
Suboptimal quality of antimicrobials
OIE research in more than 130 countries found that most LMIC countries lack legislation for the control and regulation of veterinary products. Countries with regulatory frameworks on antimicrobials often have problems with implementation and enforcement due to financial constraints. Without effective regulation, poor quality and counterfeit antimicrobials, and uncontrolled antimicrobial sales are likely to be problematic (OIE, 2017).
1.4 AMU in companion animals
The amount of antimicrobials used in pets is significantly lower than in food-producing animals. However, because they live in close proximity to humans, AMU in pets may be important for AMR transmission. Antimicrobials commonly used in humans are often used in companion animals, too.
The active ingredients in human and animal antibiotic preparations are often similar or identical. How is this relevant to the emergence of AMR?
The main risk is that administering human medicines to animals is likely to drive the emergence of resistance to those drugs, which could make it difficult to treat human patients with bacterial infections. This topic will be described further in Sections 2 and 3.
1.5 Drivers of AMU
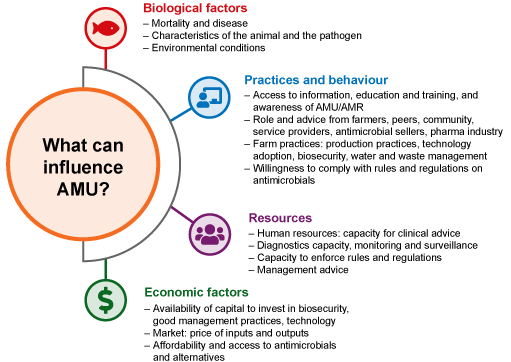
AMU and practices in food-producing animal systems are influenced by human behaviour, as shown in Figure 3. Information about the drivers and motivations for using antimicrobials in animal production systems can be helpful in planning action to reduce AMU. The details vary in different settings.
Activity 2: Understanding and reflecting on AMU in your own setting
Understanding AMU in your own setting
- Choose a food animal production system you are familiar with; for example, broiler chickens, shrimp or dairy cattle. Draw a simple diagram of this production system, outlining the production stages needed to grow the animals to food age, and add inputs to the system such as food, water and medicines.
- Indicate on your diagram where antimicrobials might be used in the system.
Note that you will need to refer this diagram in later activities.
Discussion
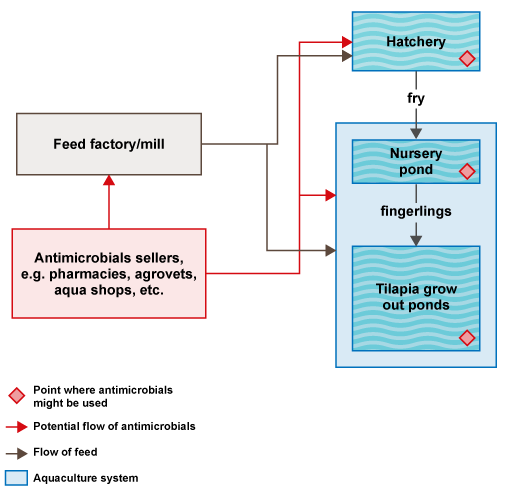
Figure 4 is an example of a section of a
Activity 3: Decision-making about antimicrobials in your setting
How are decisions made about AMU in your setting? Who is involved in decision-making? Are diagnostic tests used to identify the pathogen before using antimicrobials? Write your answer in the space below.
Discussion
Your answer will be specific to your setting. Ideally, a qualified animal health professional such as a veterinary surgeon should be involved in decisions about AMU. Bacterial infection should be confirmed and the bacteria would be tested for AMR before using these drugs, but this type of laboratory testing may not be undertaken in your setting.
2 AMR in animal food systems
In this section we discuss how using antimicrobials in animals drives the emergence of resistance, and how resistance can spread between humans, animals and the environment. The links between animal health, human health and the environment are also discussed in more detail in the Introducing a One Health approach to AMR module.
After completing this section you will be able to:
- describe the main mechanisms by which AMR in animal production systems may influence the occurrence of resistance in human pathogens, and other routes influencing the occurrence of resistant bacteria in animals and the environment
- illustrate the links between animal health, human health and the environment in animal production systems in relation to AMR
- apply scientific terminology related to AMR when explaining your current work.
2.1 Introduction to AMR in animal health
Any use of antimicrobials in animals increases the risk of AMR emerging, although the risk is enhanced by the factors outlined at the end of Section 1.2.
2.1.1 Use of antimicrobials in animals selects for resistance
The ways in which the use of antimicrobials in animals can influence the emergence of AMR are illustrated in Figure 5.
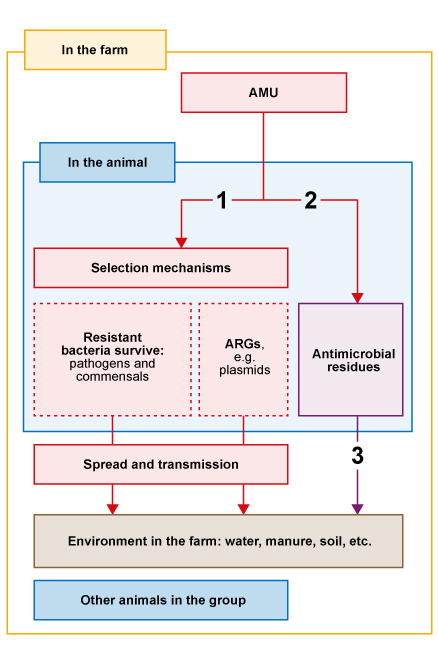
The three pathways in Figure 5 act as follows:
- AMU speeds up the selection of resistant bacteria and
antimicrobial resistance genes (ARGs) . In the animal, bacteria with genes that confer resistance to the antimicrobial administered will survive when they are exposed to that antimicrobial. Resistant bacteria multiply in the animals, especially in the gut. Resistant bacteria and ARGs are shed into the environment where they can spread to other animals by routes such as direct contact and faeco-oral transmission. Transmission is more likely in conditions of high stocking density and poor hygiene. - Antimicrobials given to animals can persist as breakdown products or
residues in the animal for varying lengths of time, depending on the specific drug, dose and route of administration. Thewithdrawal period is the minimum period of time allowed between the last administration of a specific drug to an animal, and the collection of edible tissue or products from the animal. It ensures that the residues in food comply with the maximum residue limit for that specific drug (Codex Alimentarius, 1993). - In addition, antimicrobials may be excreted in the urine or faeces to the environment. Excretion can be due to incomplete absorption of oral drugs, or excretion of drugs (or their
active metabolites ) given by non-oral routes. These excreted drugs or their metabolites are known as residues. An active metabolite is a version of the drug that has been chemically changed by the body but still has some antimicrobial properties.
2.1.2 Different types of bacteria and their roles in AMR emergence
How can genes for resistance spread between bacteria? (This is a reminder of content explained in the Introducing antimicrobial resistance module.)
Resistance to antimicrobials can be spread by
vertical gene transfer of mutations from parent to offspring bacteria (where offspring will have the same genetic mutations as the parent), andhorizontal gene transfer , or the acquisition of new genes from other bacteria – the main mode of transfer of resistance to antimicrobials.
It is typical for animals to be exposed to bacteria in the environment. They also carry a substantial and diverse population of microbes on their skin and in their guts: the
Bacteria can be classified as
Bacteria that can be transmitted between animals and humans and cause infection are called
| Example | Animals | Humans |
|---|---|---|
| Campylobacter spp. | Commensal (poultry) | Pathogenic |
| Non-typhoidal Salmonella spp. | Commensal (poultry) | Pathogenic |
| E. coli | Commensal (most strains) | Commensal (some strains may be pathogenic) |
| Enterococcus spp. | Commensal (most strains) | Commensal (some strains may be pathogenic) |
| Mycobacterium bovis | Pathogenic (cattle) | Pathogenic |
The digestive tracts of humans and animals contain thousands of millions of bacteria, most of them commensal. When antimicrobials are administered to animals, some of the commensal bacteria die. Certain commensal bacteria that are usually present in small numbers (such as Escherichia coli, Salmonella spp. or Campylobacter spp.) and that may contain resistance genes can survive and replicate, outnumbering the rest of the commensal bacteria, and becoming a dominant component of in the gut microbiota.
Both pathogenic and commensal bacteria can carry ARGs and can be transmitted to humans. Once resistant bacteria reach the human gut, resistance genes can be transferred between commensal and pathogenic bacteria. Commensal bacteria can act as significant reservoirs of AMR genes in the gut microbiome and the environment. Humans, animals and the environment can be reservoirs of resistant bacteria, which are themselves reservoirs of ARGs. Sources of resistant bacteria, residues and ARGs in the environment could be waste systems such as manure heaps, hospital or pharmaceutical plant waste, the soil and water courses (Toutain et al., 2016).
Note that a reservoir of AMR is defined as any component of a system that can contain resistant bacteria or ARGs.
Choose the two correct statements about different types of bacteria:
a.
Commensal bacteria are harmful to the host.
b.
Zoonotic bacteria always cause disease in animals.
c.
Zoonotic bacteria can be transmitted from animals to humans, where they may cause disease.
d.
Pathogenic bacteria usually only cause disease in one host species.
e.
The digestive tract of animals and humans contains a wide range of commensal bacteria.
The correct answers are c and e.
The case of E. coli as commensal bacteria and indicator
E. coli is considered a commensal zoonotic bacterium common to animals, humans and the environment, and commonly acquires AMR genes. Some of these genes control the production of an enzyme, called
E. coli is usually a commensal for animals, but some strains may be pathogenic to humans. If those strains also have the ESBL enzyme, they will be much more difficult to treat. ESBL-E. coli has been used as a representative indicator organism, which means that its presence in different settings is monitored to give us an indication of the magnitude of the AMR problem.
2.2 Types of human exposure to antimicrobials and resistant bacteria originating from animal systems
Human exposure to antimicrobials leads to selection for resistant bacteria in humans. Humans can be exposed to resistant bacteria, ARGs and antimicrobials in three main ways, as discussed below.
Transmission by direct contact: occupational and recreational
Resistant bacteria can be transmitted to humans by direct contact with animals or animal products. Some occupations are at higher risk of exposure if appropriate biosecurity, hygiene and control measures are not followed. People at risk of occupational exposure include farmers, who might be exposed to antimicrobials when administering them to animals, and also abattoir workers.
Transmission of resistant bacteria can also occur by direct contact during recreational activities between humans and animals. Studies have shown that pets can be reservoirs for resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant E. coli and Salmonella.
Consumption of foods of animal origin: foodborne transmission
Foods of animal origin such as meat, fish, eggs and milk can contain pathogenic and commensal resistant bacteria, or residues.
If food safety guidelines are not properly followed during food chain processes, animal products can be contaminated with resistant bacteria from other products, or from workers with resistant bacteria. Also, if animal products are not stored properly, bacteria (resistant or not) can multiply. Consumers of these products will then be exposed to resistant (and non-resistant) bacteria that can be transmitted by food unless properly cooked. If the withdrawal period has not been followed, humans could be exposed to antimicrobial residues in food, which might select for resistant gut bacteria.
Environment: waterborne and indirect transmission
Resistant bacteria, ARGs and antimicrobial residues from food animal producing systems can contaminate water courses through animal waste, wastewater, sediments, manure or sewage water. Soil may also be contaminated by animal manure or aquaculture pond sediments. Waste from companion animals and wildlife can also contaminate watercourses and soil. Humans can then be exposed by contact with the contaminated water or soil, for example by swimming or drinking the water.
2.3 Activities that increase the risk of AMR spread
A number of other factors are important in the emergence of resistance and spread of resistant bacteria and ARGs between humans, animals and the environment. It is important to note that resistant bacteria can be transmitted from humans to animals and vice versa. We have included some examples of key activities that promote AMR below.
Food animal production systems
In many countries it is common to use manure from poultry or pig farms to fertilise aquaculture ponds. Also, farmed ducks may swim in aquaculture settings. This practice can lead to the spread of resistant bacteria, ARGs and antimicrobial residues that can select for resistance in the aquaculture setting.

Hospitals and antimicrobial manufacturing
Wastewater and sewage from hospitals can contain high levels of resistant bacteria, ARGs and antimicrobial residues. Patients excrete bacteria in faeces and they may also excrete antimicrobial residues if they have received antimicrobial treatment. Antimicrobial manufacturing sites can also be hotspots for AMR spread, due to the potential release into the environment of ingredients that can select for resistance (Berendonk et al., 2015).
In the absence of water and waste treatment management, these human products can contaminate water courses and reach food producing animal systems. Recent studies have shown that ARGs in aquatic systems may be derived from human sources (Rowe et al., 2016).
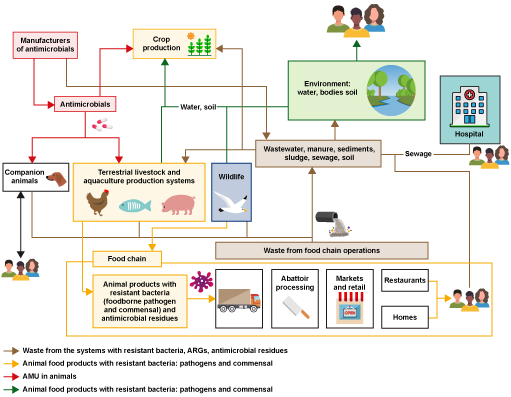
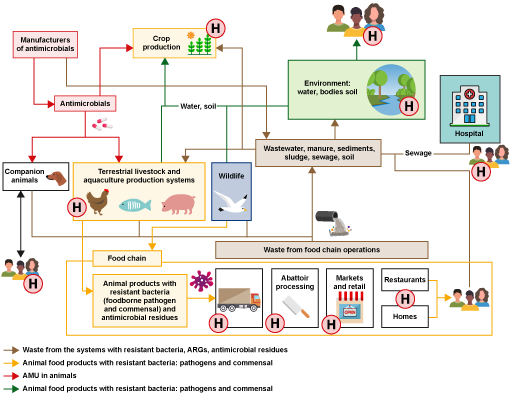
Figure 9 provides an overview of how AMR can spread, and the exposure types and transmission pathways that can lead to exposure of humans, animals and the environment to resistant bacteria, ARGs and antimicrobial residues, as explained in Sections 2.2 and 2.3. This figure can be used to identify activities that influence the emergence and transmission of resistant bacteria. For example, start with ‘antimicrobials’ near the top left of the diagram. The red arrows show that they can be administered to food animals and then the yellow arrows show that the resulting food products with resistant bacteria and antimicrobial residues can enter food chain operations such as abattoirs. The brown arrows show that waste from food chain operations can enter the environment, affecting soil, water and sediments (green arrows). Spend some time exploring the rest of the figure.
Activity 4: Types of exposure and transmission pathways
Looking at Figure 9, consider which points in the system might be particularly important for human exposure to resistant bacteria and antimicrobial residues (these could be described as ‘hotspots’). Use the space below to list the hotspots before looking at the discussion.
Discussion
There is a wide range of pathways by which humans can be exposed to resistant bacteria, ARGs and antimicrobial residues. Key points for human exposure are shown in Figure 10 – don’t worry if you didn’t identify all of them.
Activity 5: Human exposure and AMR transmission in your setting
Use the food production diagram you created in Activity 2 to complete this activity on paper or on a computer.
- Using the links you learnt about in Sections 2.2 and 2.3, add as many of them to your diagram as you can to show where resistant bacteria may be transmitted between animals, humans and the environment.
- Use a specific symbol (such as an H) to indicate where on your diagram humans might be exposed, including different types of occupational exposure and considering all relevant stakeholders.
Hint: remember to consider occupational, foodborne and environmental exposure.
Discussion
Occupations that you might have considered to be exposed to AMR include:
- farm workers in direct contact with the animals
- waste disposal workers, e.g. removing carcasses from the farm or removing faeces for fertiliser
- abattoir workers in contact with animal carcasses and organs contaminated with resistant bacteria.
- food processing workers who are in contact with animal products
- food retailers, e.g. at a market stall or shops
- veterinarians
- officials involved in e.g. farm and abattoir inspections.
The next section has an example of the type of diagram we had in mind; however, your diagram will be specific to your setting, and may be considerably simpler or laid out differently.
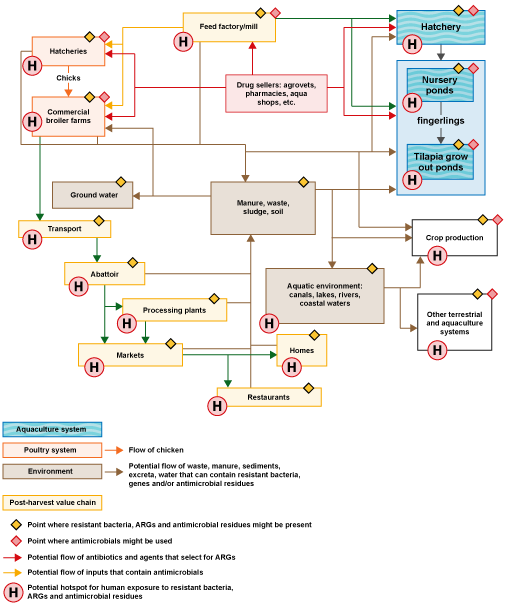
2.4 An example of AMR transmission in a food production system
Figure 11 shows the same system used as an example in Activity 2, but we have developed the diagram to show how AMR can be transmitted by the routes discussed earlier in this section. Some routes are not shown on the figure; for example, tilapia would also enter the food chain. We have indicated some hotspots for human exposure but there are other routes.
3 Consequences of AMR for animal health, public health and the environment
In this section we explain how AMR in animals affects animal health, food production, human health and the environment.
After completing this section you will be able to:
- explain the consequences of resistant bacteria in animals for animal health, food production and human health
- illustrate the links between animal health, human health and the environment in animal production systems in relation to AMR
- apply scientific terminology related to AMR when explaining your current work.
3.1 The impact of AMR
This section looks at the ways in which resistant bacteria in animals can have an impact on all aspects of life.
3.1.1 Animal health and food production
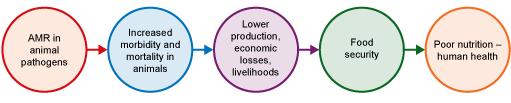
As explained in Section 1, antimicrobials are used in animals to control pathogens that cause disease, and therefore production losses. Any use of antimicrobials creates selection pressure, leading to dominance of resistant bacteria. Antimicrobials are less effective when resistant bacteria dominate, causing higher morbidity and mortality in animals due to untreatable bacterial infections. Production losses and expenditure on managing resistant infections in animals affect the livelihoods of farmers and other stakeholders in food production systems (Rushton, 2015).
AMR in animal pathogens can affect food supply by reducing the numbers of animals reaching slaughter or harvest weight. This reduced availability can lead to increased prices for animal products, putting them beyond the reach of the poorest people. An inability to access these nutritionally valuable but more expensive foods could contribute to poor nutritional health in affected people, and this is expected to particularly impact LMICs. For more details of the links between AMR in animal pathogens and food safety and security, and also the balance between benefits and adverse effects of AMU in animals, we strongly encourage you to read ‘Anti-microbial use in animals: how to assess the trade-offs’ (Rushton, 2015).
3.1.2 Human health and associated socio-economic impact
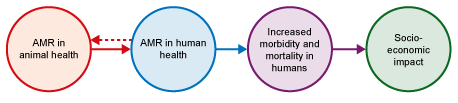
AMU in food-producing animals contributes to AMR in human health by the types of exposure discussed in Section 2.2, as shown in Figure 13. Development of resistant infections in humans due to use of antimicrobials in animals is thought to be a small fraction of the total, but is not easy to quantify.
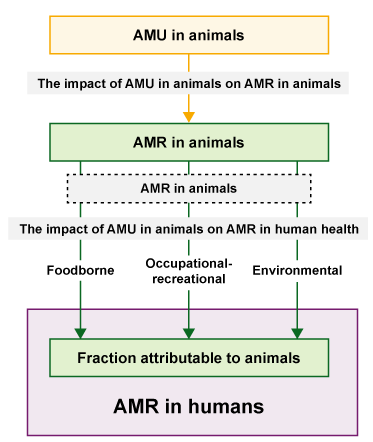
AMU and resistant bacteria in animals contribute to resistant infections in humans through the exposure pathways, as explored in Section 2.2. As explained in Section 2.3, resistant bacteria and ARGs can also be transmitted from humans to animals and animal products. Increased AMR in human pathogens leads to difficulty treating bacterial infections and an increase in human morbidity and mortality. Resistant pathogens are estimated to cause 700,000 human deaths per year globally and deaths are expected to reach 10 million per year by 2050 if no action is taken (O’Neill, 2016). Resistant infections in humans have a socio-economic impact due to, among others, the cost of longer hospitalisations. There are also indirect societal costs relating to long-term disabilities or reduced earning ability with associated impact on families.
3.1.3 Environmental impact of AMR
Selection pressure and microbial diversity
As explained in Section 2.1.1, animals treated with antimicrobials only absorb a small fraction (5–15%) and excrete antimicrobial active metabolites in the faeces that enter the environment and may select for resistant bacteria, reducing the overall microbial diversity.
In aquatic environments, a selection pressure is created when antimicrobials are mixed with feed and added to aquaculture ponds or rivers. Any of the drug not consumed by the fish is present in the water, reducing microbial diversity there. This change could have an adverse impact on the bacterial communities and nutrients needed for healthy agriculture systems. Antimicrobials not consumed by the fish can enter rivers, coastal waters and marine environments causing further disruption to distant ecosystems.
Influence of climate change
Recent research has demonstrated the potential relationship between AMR in aquaculture and global warming. Increases in temperature can adversely affect fish immune systems so warmer temperatures may contribute to more outbreaks of bacterial diseases in fish, leading to increased use of antimicrobials. Research also shows that the amount of resistance in aquaculture systems is related to climate variability, which would be more significant in climate-vulnerable countries (Reverter et al. 2020).
3.2 Antimicrobial residues
Although the implications of antimicrobial residues in the environment are not fully understood, they can affect human (and animal) health by causing allergic reactions and changes to human or animal intestinal flora. Antimicrobial residues also influence the emergence and subsequent spread of resistant bacteria by acting as a selection pressure.
In food production, the presence of residues in animal food products destined for export can lead to products being rejected with resulting economic impact.
Activity 6: The impact of AMR in your sector
Look again at the animal production system that you chose in Activity 2 and your diagram showing exposure pathways in Figure 11.
Can you identify the potential consequences of AMR in animal systems for public health, animal health, food production and environment in your chosen system? Think about:
- animal health and welfare, and food production
- human health
- the environment.
Discussion
Your examples will be specific to your setting, but the ideas below are based on the model scenario we provided in the previous section of commercial broiler and tilapia:
- Animal health and welfare, and food production: Higher morbidity and mortality due to resistant infections in poultry and fish that cannot be treated with antimicrobials would lead to lower productivity, higher expenditure in alternative measures to tackle bacterial infections and economic losses for the farmers.
- Human health: Consumers of food products containing resistant bacteria, people exposed to water sources or food chain workers could be infected with resistant zoonotic bacteria and need to undergo treatment.
- Environment: If the waste and water from the food chain operations are not treated, they would go into the environment, contaminating watercourses. There could be loss of diversity due to the antimicrobial residues that select for resistant bacteria.
4 Using data to tackle the AMR crisis
This section briefly introduces different types of data relevant to AMR, and how they can help to address it. The concepts discussed in this section will be covered in more detail in the AMR surveillance in animals module.
After completing this section you will be able to:
- explain why monitoring AMR in food animal systems (including samples from healthy animals) is critical for tackling the AMR crisis
- apply scientific terminology related to AMR when explaining your current work.
4.1 Monitoring and surveillance of AMU/AMR
The use of antimicrobials and the presence of resistant bacteria and antimicrobial residues can be monitored regularly, providing necessary information for AMR surveillance systems.
Ideally, monitoring and surveillance systems should consider both sick and healthy animals to look for disease and carriage of resistant bacteria.
4.2 AMU data
AMU is the quantity of antimicrobial drugs prescribed or administered to an individual person or animal, or group of animals (such as a herd or flock), measured at the level of healthcare facility, farm, region or country and typically measured as kg of antibiotics. Data on the use of antimicrobials is required, for example, to be able to estimate the effects of AMU on
Ideally, AMU data collected at the farm level should report:
- the exact dose of active antimicrobial administered
- the number of animals that receive it
- exact reasons for administration, as described in Section 1.3.
Instead, the information usually available is antimicrobial consumption (AMC) based on sales or imports data, as defined in Section 1.1.
The OIE is coordinating efforts to harmonise data collection on the quantities of antimicrobial agents intended for use in animals. Currently this approach measures AMC, but the OIE aims to capture AMU data in future. The OIE produces a yearly report based on data supplied by country members; the latest report shows data from 2014 to 2016.
Figure 16 shows an example of AMU data reported in pig production in Thailand and Vietnam. The data was obtained as part of a study investigating the use of antimicrobials in pigs, poultry and aquaculture production systems.
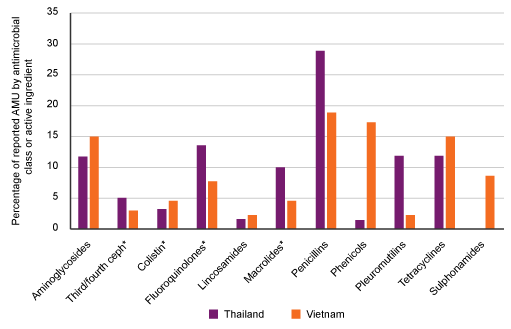
Activity 7: Interpreting usage data
Use Figure 16 to answer the following questions:
- Which type of active ingredient was used most commonly and least commonly in each country?
- How could this information play a part in tackling the AMR crisis?
Answer
Penicillins were most commonly used in both settings (approximately 28% of reported usage in Thailand and 18% in Vietnam).
Lincosamides (e.g. clindamycin) and phenicols (e.g. chloramphenicol) were least commonly used in Thailand (both approximately 2% of reported usage).
Lincosamides and pleuromutilins were least commonly used in Vietnam (both approximately 2% of reported usage).
There are many possible answers, but key points are:
- as a baseline to monitor AMU over time (for example, annually), which would allow the impact of reduction strategies to be analysed
- to set appropriate targets for reduced use of different types of antimicrobials
- to target educational programmes around commonly used antimicrobials to effectively reduce use
- reducing selection pressure by antimicrobials on bacteria should reduce the prevalence of resistant bacteria over time
- lower prevalence of antimicrobial resistance will reduce the consequences of AMR (as discussed in Section 3) seen in the country.
4.3 Data on resistant bacteria
Data on resistant bacteria, both pathogenic and commensal, are extremely important for understanding the impact on public health and for an effective response to the problem. Monitoring and surveillance systems for resistant bacteria need to be in place to generate these data. Information about changes in resistant bacteria over time, such as changes in prevalence, is necessary to understand the effect of any interventions. The FAO is coordinating the future harmonisation of AMR monitoring and surveillance data in animals.
Data collection on resistant bacteria must include both sick and healthy animals. As you have learnt earlier in the module, resistant commensal bacteria do not cause clinical signs in animals, but some of them may be transmitted to humans where they could cause disease. Monitoring and surveillance systems are needed to assess the new emergence of resistance in animals and the effect of interventions.

Data on resistant bacteria can be obtained through a variety of methods, depending on the purpose of the investigation. For example,
Samples for both types of tests could be taken from a range of sources, such as sick and healthy animals, carcasses, other animal products, animal feed and the environment. In the broiler and aquaculture system shown in Figure 11, we could investigate potential transmission of resistant bacteria in the system by testing for non-typhoidal Salmonella spp. in broiler faeces, water from the aquaculture pond and carcasses of broilers at the abattoir. The results from each part of the system could be compared to each other as well as to data obtained from the local hospital.
4.4 Antimicrobial residue data
Carcasses and animal products (such as milk samples) can also be tested for the presence of antimicrobial residues. Residue data can also be collected using samples from abattoirs, domestic markets and processing plants. Environmental samples such as wastewater from a broiler farm could also be useful for residue testing.
Match the following specific benefits to either AMU surveillance and reporting, or data on resistant bacteria.
‘Allows patterns of AMU to be identified.’
a.
AMU surveillance and reporting
b.
Data on resistant bacteria
The correct answer is a.
‘Allows comparison with AMR patterns in humans to analyse possible transmission.’
a.
AMU surveillance and reporting
b.
Data on resistant bacteria
The correct answer is b.
‘Informs decision-making for the effective treatment of unwell animals.’
a.
AMU surveillance and reporting
b.
Data on resistant bacteria
The correct answer is b.
‘Provides a baseline allowing targets to be set to reduce AMU.’
a.
AMU surveillance and reporting
b.
Data on resistant bacteria
The correct answer is a.
Explain the benefits of testing bacteria from asymptomatic animals for AMR – perhaps using a specific context, such as a broiler unit.
Testing healthy animals can identify the presence of resistant commensal bacteria, which can be transmitted to humans and to the environment. This information can inform the choice of antimicrobial used for animals in that setting in future. It can also allow targeting of strategies to tackle AMR, for example, targeting reduction of use of a particular antimicrobial. Changes in resistant bacteria in asymptomatic animals over time can reflect the effectiveness of strategies to tackle AMR.
Activity 8: Data on resistant bacteria in your setting
Return to the diagram of an animal production system you used in Activity 5 to answer the following questions:
- Choose at least three points in the system that could be useful sources to investigate the presence of resistant bacteria using the types of testing described in Section 4.3.
- For each example, mention the purpose of the data you have chosen – that is, how that information could be used. Examples of different purposes would include:
- guiding the treatment of sick animals
- investigating the presence of resistant commensal bacteria
- investigating transmission of resistant bacteria between different parts of the system.
- For each one, choose the specific sample you would send to the laboratory for testing. Assume that all samples will be cultured to isolate the target bacteria. Genotypic techniques would then be used to identify the presence of resistance genes and to compare strains found in different sources.
We have provided a blank table template for you to complete your plan. The template includes an example from the production system example in Figure 11 as a guide. Add at least three examples from your own setting. We have provided two extra rows for any further examples that you would like to add; if you like, you could add more rows (or continue on paper) if you have additional examples.
Discussion
Your answer will be specific to your setting. Table 2 includes three examples based on the production system shown in Figure 11. (Revisit Section 4.3 if you need to revise this topic.)
| Name | Source | Purpose | Sample |
|---|---|---|---|
| Our example | Healthy broilers from the broiler farm | To monitor the emergence of resistance, especially in commensal bacteria | Faeces from the broiler shed |
| Our example 1 | Pond water and sediments from the aquaculture ponds | To establish the presence of resistant bacteria and compare with the data from broilers to investigate possible transmission | Water and sediment samples |
| Our example 2 | Animal feed | To check whether the feed is contaminated with resistant bacteria | The broiler feed provided by the feed company |
| Our example 3 | Sick fish from an aquaculture pond where some fish have died | To confirm whether pathogenic bacterial infection is present and to guide choice of antimicrobial | Organs from fish carcass(es) |
| Extra example | The environment | To establish the presence of resistant bacteria | Abattoir wastewater samples |
| Extra example |
Activity 9: Benefits of coordinated testing for resistant bacteria
Look at the table you created in Activity 8 about data on resistant bacteria. Explain why all three (or more) sources of data are more useful than just one of your examples.
Discussion
Your response will be specific to your setting. Based on our example table, we could have chosen to suggest the following:
- Testing sick or dead fish helps to guide treatment, but on its own does not allow analysis of transmission of AMR in the system.
- Testing both broilers and pondwater allows comparisons of resistant strains in both parts of the system.
- If the same resistant strains are found in broiler feed and in the healthy broilers, the feed may be an important source of resistant bacteria in the broilers, which could be addressed by improving hygiene in the animal feed process.
- Comparing resistant bacteria in abattoir wastewater and in fish farm samples such as pondwater could help to demonstrate transmission of resistant bacteria from the environment to aquaculture ponds via contaminated water sources. Molecular techniques could be useful here.
5 End-of-module quiz
Well done – you have reached the end of this module and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
6 Summary
In this module you have learned how and why antimicrobials are used in animals. You have investigated how animals can become infected with or carry resistant bacteria and ARGs, which can spread and be transmitted to other parts of a food-producing system, including humans and the environment.
You have explored the consequences of AMR for animals, humans and the environment. You have been introduced to three types of data needed to address the AMR challenge. Throughout the module you have had opportunities to reflect on AMR in an animal industry setting relevant to you.
You should now be able to:
- describe the ways in which antimicrobials are used in animals
- describe the main mechanisms by which AMR in animal production systems may influence the occurrence of resistance in human pathogens, and other routes influencing the occurrence of resistant bacteria in animals and the environment
- explain the consequences of resistant bacteria in animals for animal health, food production and human health
- explain why monitoring AMR in food animal systems (including samples from healthy animals) is critical for tackling the AMR crisis
- illustrate the links between animal health, human health and the environment in animal production systems in relation to AMR
- apply scientific terminology related to AMR when explaining your current work.
Now that you have completed this module, consider the following questions:
- What is the single most important lesson that you have taken away from this module?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 10: Reflecting on your progress
Do you remember at the beginning of this module you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this module, go to your reflective blog and note down your thoughts.
7 Your experience of this module
Now that you have completed this module, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this module and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Acknowledgements
This free course was collaboratively written by Maria Garza and Isobel Richards, and was reviewed by Lucy Brunton, Claire Gordon, Natalie Moyen and Hilary MacQueen.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Course image: Deyana Robova/123RF.
Figure 1: davit85/iStock/Getty Images Plus.
Figure 2: Mark Hunt/Getty Images.
Figure 6: Steve Gschmeissner/Science Photo Library.
Figure 7: tanakornsar/Shutterstock.
Figure 8: Gabrielle Vonot/Look At Sciences/Science Photo Library.
Figure 13: adapted from Innes et al., 2020. This file is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence, https://creativecommons.org/ licenses/ by/ 4.0/.
Figure 15: Thitipong Kingkaeo/123RF.
Figure 16: from Coyne et al., 2019. This file is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence, https://creativecommons.org/ licenses/ by/ 4.0/.
Figure 17: Duangphorn Wiriya/Unsplash.
Figure 18: used with permission of Daniela Beckmann, https://www.flickr.com/ photos/ _dane/ 23921316659.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.