Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 28 April 2024, 6:25 AM
Antimicrobial stewardship in animal health
Introduction
Welcome to Module W of this course. This module is aimed at animal health professionals including laboratory professionals, veterinary services and managers, and policymakers.
The term ‘stewardship’ means the responsible management of something that has been put into someone’s care. And so, when we ask someone to be an ‘antimicrobial steward’, what we are really asking is that they use
In this module, you will learn about
While antimicrobials are used in most animals, the quantities used in livestock (e.g. chickens, pigs, cattle) and aquaculture are of most concern for public health. Therefore, we will focus our learning on AMS in livestock and aquaculture.
In this module, the term ‘
Please be aware that this module is longer than most, involving eight hours of study.
By the end of this module, you should be able to:
- define the five principles of
AMS in animal health - describe how
intrinsic andextrinsic factors drive prescribing behaviour - list and explain the different
therapeutic andnon-therapeutic uses of antimicrobial agents in food animal production - identify the relationship between AMS and animal welfare
- list antimicrobial agents rated as critically important for people that are commonly used in food animal production.
Activity 1: Assessing your skills and knowledge
Before you begin this module, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Do not worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these modules.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
1 Use of antimicrobials in animal health
1.1 Purposes of antimicrobial use in food animals
Antimicrobials have been used in animals for almost as long as they have been available to people. They have revolutionised our approach to the treatment and prevention of diseases and have brought considerable benefits to people and animals by:
- alleviating pain and suffering
- ensuring
food security - protecting livelihoods
- reducing zoonotic disease spread.
However, as
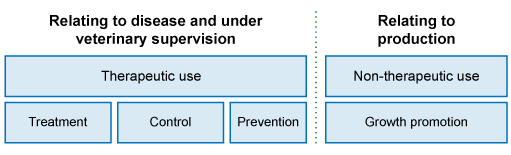
Antimicrobials are used in food animals for a range of purposes, which broadly fall into two categories: therapeutic uses to control diseases and non-therapeutic uses (Figure 1). In the following sections, we will review these purposes in more detail.
Veterinarians are qualified and registered or licensed by a government veterinary statutory body to practise veterinary medicine (or veterinary science) in the country in which they are registered. In almost all countries, veterinarians are responsible for prescribing antimicrobials for use in animals.
Veterinary
1.1.1 Therapeutic uses of antimicrobials in animals
‘Therapeutic use means the administration of an antimicrobial agent to an individual or group of animals to treat, control or prevent infectious disease’
Even with good
Definitions for therapeutic uses of antimicrobials in animals are provided in Table 1.
| Term | Definition |
|---|---|
| Treatment | Administration of an antimicrobial to an individual or a group of animals showing clinical signs of an infectious disease. Situations where antimicrobials may be used in clinically sick animals are many and varied. Common bacterial diseases in food animals that often require antimicrobials include mastitis, |
| Control | Administration of an antimicrobial to a group of animals including sick and healthy animals (presumed to be infected), to minimise or resolve clinical signs and to prevent further spread of the disease. Control is sometimes called Situations where antimicrobials may be used for control include bacterial respiratory diseases in intensively reared animals, post-weaning diarrhoea, |
| Prevention | Administration of an antimicrobial to an individual or a group of animals at risk of acquiring a specific infection or in a particular situation where infectious disease is likely to occur if the drug is not administered. Prevention is sometimes called Situations where antimicrobials may be used when an animal is not currently diseased include giving an antimicrobial after surgery or traumatic injury, when animals are under a lot of stress such as being raised in a feedlot to prevent respiratory disease or foot infections, dry cow therapy for dairy animals, or to prevent liver abscesses caused by high-grain diets. |
Ideally, antimicrobials should only be prescribed for therapeutic purposes by a veterinarian or a trained person (e.g, veterinary paraprofessional) under the supervision of a veterinarian, according to national laws (OIE, 2019).
However, the reality in many low- and middle-income countries (
1.1.2 Non-therapeutic uses of antimicrobials in animals
‘Non-therapeutic use means the administration of antimicrobial agents to animals for any purpose other than to treat, control or prevent infectious disease’
It is well established that antimicrobials contribute to the rapid production of food animals. For this reason, farmers may want to use antimicrobials for non-therapeutic purposes. The most common non-therapeutic uses of antimicrobials are to promote growth and increase feed efficiency. Another example of non-therapeutic use of antimicrobials is
You may have noticed from Table 1 that the OIE defines ‘prevention’ as a therapeutic use. The inclusion of ‘prevention’ in therapeutic uses is contentious among public health groups. This is because many groups, including the WHO, view prevention as a non-therapeutic use of antimicrobials (WHO, 2017b). The practice is seen as a poor substitute for good husbandry, hygiene, and biosecurity on farms. There is also a strong belief in scientifically informed circles that antimicrobial use for the prevention of diseases is unnecessary and increases the burden of AMR in animal populations. For those working in animal health, prevention is seen as important for animal health and welfare: in other words, ‘prevention is better than cure’ and thus, we have an apparent contradiction.
According to the OIE, the use of antimicrobials for preventative purposes should only be done under veterinary supervision (OIE, 2019). However, this is not always possible in LMICs, where there is often widespread access to antimicrobials from non-veterinary suppliers such as agrovet stores without a prescription.
1.1.3 Antimicrobials used for growth promotion
‘Growth promotion means the administration of antimicrobial agents to animals only to increase the rate of weight gain or efficiency of feed utilisation.’
In the 1940s, antimicrobial waste products fed to chickens were found to have growth promotion effects. Since then, antimicrobials have been routinely added to animal feed to increase productivity. For a long time, sub-therapeutic levels of antimicrobials in feed were believed to be a nutritional supplement.
The concern with using
- Dosing is at sub-therapeutic levels
- Consumption of medicated feed is relatively uncontrolled in that individual animals consume varying amounts of feed.
This creates an ideal environment for the selection of resistance in

Once it became apparent AGPs contributed to AMR in bacteria carried by animals, nations began imposing regulatory restrictions and prohibitions on their use, with the European Union (EU) beginning the trend in 1997. Today, many countries, including many LMICs, have banned the use of AGPs. According to the latest OIE report on antimicrobial use in animals, 77% of responding countries indicated they did not use any AGPs in animals, either with or without regulatory provisions to impose the demands (OIE, 2020).
There have been unintended consequences from banning AGPs in food animals. After the AGP bans came into effect in the EU, the trend for using antimicrobials for preventative purposes increased. The prohibition of AGPs showed up weaknesses in animal husbandry and biosecurity practices on farms. The AGP bans in the
Not all AGPs are controversial. For example, the
People who advocate for AGPs in animals argue that their use is critical for food security and the economic viability of individuals and nations reliant on agriculture. However, others say that caution should be taken with the continued use of antimicrobials in food animals, given the risk posed to human health. One thing is universally agreed upon: alternatives to AGPs such as vaccination, improved nutrition, hygiene and biosecurity are urgently needed.
Activity 2: Opportunities and innovations in animal health that are mindful of the AMR burden
Take some time to reflect on how you think we can best balance the growing demand for animal protein in LMICs with the projected expansion of intensive farming systems and associated increase in AMU. Are there opportunities and new innovations that can be adopted in animal health sectors that can match supply and demand that don’t increase the overall burden of AMR in animal populations?
Discussion
There are many long-standing practices that help to reduce the burden of animal diseases on farms. For example, implementing strict biosecurity and hygiene practices helps to keep infectious diseases out, while employing good husbandry practices like preventing overcrowding and providing good quality feed and water, improves the overall health and welfare of animals. Using antimicrobial treatment alternatives such as vaccines can reduce the disease burden while probiotics can be used to improve the health and productivity of food animals.
We’ll discuss more about these opportunities to reduce the burden of AMR in food animals throughout this module.
1.2 Social licence for using antimicrobials in food animals
You might be thinking: ‘Why do I need a ‘social licence’ to use antimicrobials in food animals’?
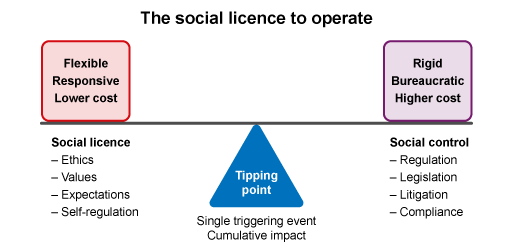
First, we need to think about what it means to have a social licence. A
If an industry has SLO, then the costs to operate are lower than an industry under ‘social control’, where governments impose a bureaucratic burden on operators (Figure 3). The tipping point between a community giving or withdrawing a SLO may be a single event (e.g. catastrophic environmental damage) or cumulative impacts over time (e.g. growing knowledge of AMR risk to people).
SLO is not new to agriculture. For example, the SLO related to animal welfare, fish farming and land clearing for crops have been widely debated for some years. Social controls have been introduced in response to these concerns, and farmers have had to develop new ways to farm.
For example, the aquaculture sector often struggles with their SLO because of opposition at local, national and international levels over the potential impact on the coastal ecology, the land rights of indigenous groups, other commercial fishers, and tourism. Broader societal concerns regarding environmental impacts can outweigh employment benefits for the local community. In the United States in 2017, a salmon aquaculture facility in Washington State collapsed, resulting in an estimated 160,000 non-native Atlantic salmon escaping into the Pacific Ocean environment. As a result of an inquiry into the collapse, and the noted failures of the company, the Washington State government phased out all non-native salmon production in their state (Mather and Fanning, 2019).
Societal pressure is being placed on the use of antimicrobials in food animals for therapeutic purposes. The WHO has published Guidelines on the use of medically important antimicrobials in food-producing animals in 2017 which make four recommendations, including the restriction of all classes of medically important antimicrobials for prevention of diseases that have not been clinically diagnosed (WHO, 2017b). Major foodservice providers have announced bans on the use of medically important antimicrobials in their supply chains or promote ‘
Societal concerns about antimicrobial use in food animals will not go away. Building community trust through responsible and judicious use of antimicrobials is essential. Trust will come in the form of adopting antimicrobial stewardship (AMS) practices and improving animal welfare, husbandry and farm biosecurity. Failure to respond to societal concerns is likely to result in tougher controls on antimicrobials (Tang et al., 2017).
Activity 3: The social licence – pros and cons
2 Access to antimicrobials
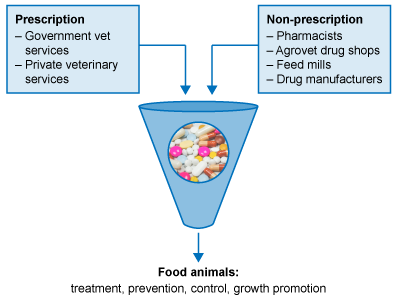
How do farmers get access to antimicrobials for their animals?
Like doctors, veterinarians can prescribe antimicrobials (but only to animals). A prescriber is someone who can give directions for the preparation and administration of a medicine used to treat a disease.
In most countries, there are laws that say only veterinarians can prescribe antimicrobials for animals. However, in some countries, prescribing laws may be weak, non-existent, and/or challenging to enforce. Also, there may not be enough veterinarians to service farmers, or the farmers are in remote locations that are difficult to get to. In these circumstances, people often access antimicrobials for their animals in other ways such as through agrovet drug stores, feed mills or in the open market (Figure 4).
2.1 Challenges in LMICs
Rapid growth in demand for animal protein has accelerated food animal production in LMICs. To meet this demand, food animal production in LMICs is shifting from small-scale landholders to large-scale and intensive farming. Small-scale farms that are expanding to intensive enterprises, and many existing intensive farms often use antimicrobials to keep animals healthy, maintain productivity and maximise economic returns.
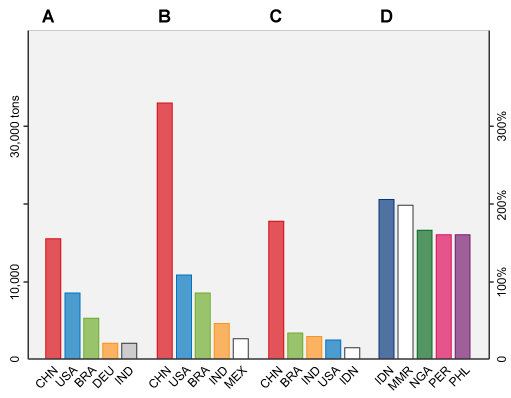
Studies have predicted that by 2030, global antimicrobial consumption (AMC) in animals will increase by 67% from 2010 levels. By 2030, the five countries with the largest share of global AMC in food animal production will be China (30%), the United States (10%), Brazil (8%), India (4%) and Mexico (2%). It is also predicted that of the 50 countries with the largest amount of antimicrobial use in food animals in 2010. Five of these, shown in Figure 5, will have the greatest projected percentage increase – Indonesia (205%), Myanmar (202%), Nigeria (163%), Peru (160%) and Philippines (157%) – in AMC in livestock by 2030 (Van Boeckel et al., 2015).
The inability to control access to antimicrobials is one of the biggest challenges facing many LMICs when responding to AMR.
In LMICs, farmers often do not have ready access to veterinary expertise, or they cannot afford their services. In such scenarios, farmers turn to other sources to access antimicrobials. Agrovet shops, open markets, online stores and feed mills fill this gap. Selling antimicrobials is an important source of income for non-veterinary services, and often little is done by governments to regulate their activities.
Access to high-quality antimicrobials is seen as a critical factor in ensuring effective management of animal health and food safety. Conversely, substandard or counterfeit antimicrobials, where the concentration of active ingredients is below therapeutic levels or a different active ingredient is used to what is on the label, are ineffective and promote AMR development.
LMICs face challenges with substandard or counterfeit antimicrobials in both human and animal health settings. An evaluation by the OIE of national animal health systems in more than 130 countries showed that many LMICs either do not have appropriate legislation or have no legislation at all to regulate the import, manufacture, distribution and use of antimicrobials in animals. The OIE also found that antimicrobials were adulterated in many of these countries (OIE, 2015).
‘Thousands of tonnes of adulterated antimicrobials destined for use in animals are circulating worldwide’
The challenge for us all is to understand how it is possible to reduce overuse and misuse of antimicrobials, including access to substandard drugs and sub-optimal dosing, especially in LMICs.
For many LMICs, where challenging farming environments prevail, antimicrobials are perceived as necessary to prevent and control animal diseases. However, many farmers have a limited understanding of AMR and AMS. Easy access to antimicrobials via non-veterinary channels poses a major barrier to adopting antimicrobial stewardship principles. Improving the regulatory capacity of LMICs to control the antimicrobial supply chain and increasing the number of veterinarians and veterinary
Activity 4: Addressing AMR – the challenges.
List some of the biggest challenges facing many LMIC responses to AMR? What can be done to address these challenges?
Discussion
Some of the biggest challenges faced by LMICs include:
- few veterinarians, veterinary paraprofessionals, and well performing laboratories available to service farming communities
- farmers getting antimicrobials and advice from non-veterinary suppliers
- inadequate knowledge about appropriate antimicrobial use
- poor quality/counterfeit antimicrobials
- poor regulatory systems (including reinforcement) to control the manufacturer, supply and use of antimicrobials in animals
- limited or no AMR and AMU data to demonstrate the scale of the issue in the animal health sector and to inform data-driven policies and practices.
Government investment in regulatory systems and in increasing the number of veterinarians and veterinary
2.2 Antimicrobials used in animals of critical importance to human health
The
| Critically important | Highly important | Important | Not used in humans | |
|---|---|---|---|---|
| Highest priority | High priority | |||
Cephalosporins (third, fourth, fifth gen.) Fluro and other quinolones Glycopeptides Macrolides and ketolides Polymyxins | Aminoglycosides Ansamycins Carbapenems and other penems Glycyclines Lipopeptides Monobactams Oxazolidinones Penicillins (natural aminopenicillins and antipseudomonal) Phosphonic acid derivatives Tuberculosis and other mycobacterial drugs | Amphenicols Cephalosporins (first and second gen.) Lincosamides Penicillins (anti-Staphylococcal) Pseudomonic acids Riminofenazines Steroid antibacterials Streptogramins Sulfonamides Sulfones Tetracyclines | Aminocyclitols Cyclic polypeptides Nitrofurantoin Nitroimidazoles Pleuromutilins | Bambermycins Quinoxalines Ionophores Orthosomycins |
The WHO CIA list serves as a benchmark for antimicrobial use in animals. The OIE also has a list of antimicrobials important to veterinary medicine. While the categories are similar, the OIE used different criteria to categorise antimicrobials than WHO. You can review the list on the OIE website.
There is considerable overlap between the WHO and OIE lists, with some of the same antimicrobial classes being categorised as critically important for both human and animal health, including:
- fluoroquinolones (FQs) – ciprofloxacin, enrofloxacin, marbofloxacin
- third and fourth generation cephalosporins – ceftiofur, Cefoperazone, cefquinome
- macrolides – tilmicosin, erythromycin, tulathromycin.
Attention is focused in both the WHO and OIE on the use of CIAs in food animals and, in particular, intensive farming. Many studies have reported instances where CIAs have been used in farming. For example:
- A survey of poultry farms in the Mekong Delta of Vietnam found that nearly a third of overall usage involved antimicrobial classes on the WHO’s HP-CIA list (Carrique-Mas et al., 2019).
- A survey of poultry farmers in Nigeria found that 80% of antibiotics used in poultry were administered without a veterinary prescription, and 63% of respondents used enrofloxacin, and 50% used erythromycin, both in the WHO highest priority group (Oloso et al., 2019).
- A study examining 94 aquaculture farms in Vietnam reported most antimicrobials used were on the WHO’s HP-CIA list, and 26.9% of fish brought from local markets tested positive for antimicrobial residues (Pham et al., 2015).
In 2017, the WHO published guidelines on using medically important antimicrobials in food animals (WHO, 2017b). You can read the report on the WHO website at your leisure.
Four recommendations were made, including:
- Reducing use of medically important antimicrobials in food animals
- Prohibiting use of medically important antimicrobials for growth promotion
- Prohibiting the use of medically important antimicrobials for preventative purposes
- Prohibiting the use of HP CIA to control or treat clinical diseases in food animals.
The WHO acknowledges there is low to very low-quality evidence to justify the recommendations. Unsurprisingly recommendations 3 and 4 have been controversial in the global animal health sector. There is widespread concern that prohibition of medically important antimicrobials will compromise animal welfare and risk more severe disease outbreaks.
Do you think medically important antimicrobials should be restricted to people only? If so, what criteria should the restrictions be based on?
Activity 5: Antimicrobial use in animals and humans
2.3 Antibiotics and animal welfare
Can you imagine what it would be like if we couldn’t treat animals with antimicrobials?
While good husbandry, hygiene, and biosecurity are critical for protecting animal health and welfare on farms, sometimes animals become sick and require antimicrobials. Veterinarians must be able to access antimicrobials to treat sick animals.
Good husbandry and welfare mean providing clean and comfortable housing, nutritious feed, clean drinking water and good air quality. These elements form the basis for keeping animals healthy, so they are more able to resist diseases. However, in many LMICs the lack of nutritious feed and clean drinking water and the sharing of common grazing areas makes livestock susceptible to disease. For many farmers, animal welfare concerns come from scarce feed and health resources rather than an absence of care.
Adopting innovative approaches to reduce long-term use of antimicrobials in food animals is likely to be more beneficial than banning medically important antimicrobials as recommended by the WHO (WHO, 2017b). Actions can include efforts to eliminate significant diseases, increase the use of vaccines, improve farming practices, and adopting AMS programmes at the local level.
3 Antimicrobial stewardship in animals
3.1 What is antimicrobial stewardship?
In the module Introducing antimicrobial resistance (module C), you learned about the mechanisms of antimicrobial agents and how bacteria develop resistance. Here we explore the importance of AMS in preventing AMR and our personal responsibilities.
Have you ever heard the phrase: ‘Use as little as possible and as much as necessary’?
AMS means different things to different people because our ideas about the concept are informed by our own experiences. As a general principle, AMS refers to the actions we take individually and collectively to preserve the effectiveness and continued availability of antimicrobials.
When people are asked what AMS means to them, their responses can be categorised into three broad themes:
- an evidence-based approach to making decisions about using antimicrobials
- judicious and sparing use of antimicrobials
- ethical and personal responsibility.
Activity 6: The concept of antimicrobial stewardship
What does AMS mean to you? What are some of the challenges and opportunities for AMS in your local context?
Discussion
AMS can mean different things to different people, and there is no absolute answer, but so long as you see that you have a role in helping to protect one of our most valuable resources (antimicrobials) then you will be aware that there are many challenges and opportunities in adopting AMS in local settings.
Many studies that have explored the barriers to adopting AMS among veterinarians and farmers have shown that a key theme to emerge is a lack of training and understanding about appropriate antimicrobial use and what it means to be responsible when prescribing or using antibiotics.
Other issues to emerge are:
- an unwillingness to change practice
- using antibiotics to make a profit
- being short on time to make a diagnosis
- fear of losing business if antibiotics are not supplied.
These issues all pose enormous challenges in the local setting. The biggest opportunity to tackle these challenges is through education of animal health officers, farmers, and other stakeholders involved in the supply and use of antimicrobials in animals. Making available free and accessible training and educational resources will help people make better decisions about antimicrobials.
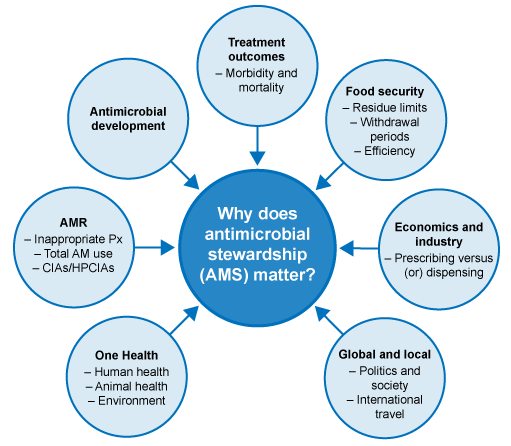
3.2 Why does antimicrobial stewardship matter?
There are a host of reasons why antimicrobial stewardship matters, from the fact it is a true
One of the first steps to take is to look closely at our motivations for using antimicrobials, both in people and animals.
Inappropriate prescribing and misuse of antimicrobials are critical problems – the more we use, the more we increase the burden of AMR. By far the biggest driver of AMR in people is prescribing in human healthcare. The same can be said for AMU and AMR in animals. But, when it comes to using antimicrobials in food animals, our actions can have an impact on human health, especially if the drugs we use are rated as critically important.
As veterinarians, paraprofessionals, farmers and regulators, we need to take up the challenge to minimise the impact of AMU in food animals as much as possible. Well thought-out and implemented AMS programmes are the means to achieve these goals.
You may have learned in other modules (The problem of AMR (module B) and Introducing AMR (module C)) that the pipeline for new antimicrobial products has dried up. Without new antimicrobials on the horizon and growing resistance to existing antimicrobials, we must be careful to preserve the resources we have left. To not do so means that more people will die from untreatable infections, which has been ominously projected to increase to 10 million people per year by 2050 (O’Neill, 2014). LMICs will most feel the impact of not managing our dwindling antimicrobial resources; they already carry the greatest burden of AMR and the loss of vulnerable people to untreatable infections.
Antimicrobial stewardship is a critical element in food security. Keeping animals healthy and free of disease while maintaining productivity and thinking about how AMU relates to these goals is an important element of stewardship. Other important elements include ensuring animal food products are free of harmful residues by following drug dosage recommendations, avoiding
3.3 Who is responsible for antimicrobial stewardship?
Everyone is responsible for AMS – from doctors, pharmacists, veterinarians, regulators and farmers down to our own choices to use antimicrobials for ourselves or families.
Watch this short YouTube video to learn about responsibilities, based on the ‘five only’ rules to handling microbials with care.
The use of antimicrobials in animals is a shared responsibility between the veterinarian and the farmer or farmworkers. In this relationship, the veterinarian takes responsibility for deciding on the most appropriate antimicrobial. The farmer is responsible for following the veterinarian’s directions and implementing good animal care practices (husbandry, hygiene, biosecurity). Together, these approaches safeguard the health and welfare of animals and help to minimise the impact of AMR in animal populations.
However, this is not the situation in all countries. As we discussed earlier, there are few veterinarians in many LMICs, and farmers access antimicrobials from multiple non-veterinary sources. Non-veterinary suppliers of antimicrobials have just as much responsibility for AMS as everyone else.
Activity 7: Applied AMS – who is responsible?
Responsibilities for AMS differ if you are a veterinarian prescribing antimicrobials, a pharmacist selling antimicrobials or a policymaker regulating antimicrobials use in food animals. Take some time to think about your responsibilities and how you can make a positive contribution to AMS in animals. Write down your thoughts.
Discussion
Did you include some of the following points when thinking about your responsibilities towards AMS?
- Keep up to date with new information and knowledge of various treatments options for diseases through ongoing education and training.
- Provide leadership or working as a team to develop an AMS programme for your place of employment.
- Share knowledge/educate farmers, agrovet sellers, co-workers, and others about AMR and AMS to help change behaviours when using antimicrobials. This includes only using antimicrobials when there is a need to use them, implementing good husbandry and farm biosecurity, getting antimicrobials from authorised sources, etc.
- Contribute to the development and evaluation of prescribing guidelines to ensure they are evidence-based.
- Promote prescribing guidelines to veterinarians and others who may use them.
- Where possible, encourage the use of laboratory testing to diagnose diseases.
- Only use antimicrobials when there is a need to use them.
3.4 Factors influencing decisions to give antimicrobials animals
While prescribing antimicrobials is relatively straightforward in human health, it can be much more complicated in animal health. Prescribing decisions are influenced by the purpose of use (therapeutic and non-therapeutic) and stakeholder needs such as cost, diagnostic testing, drug withdrawal times and social and cultural expectations.
A common outcome from seeing a veterinarian is the prescribing and dispensing of antimicrobials. There are a range of extrinsic (external) and intrinsic (internal) factors that influence decisions about using antimicrobials (Figure 7).
In countries where veterinarians and veterinary paraprofessionals are scarce, decision making about and responsibility for antimicrobial use often falls back onto farmers. In LMICs, awareness of AMR and AMS in food animals is often limited and decisions to use antimicrobials are usually based on seeking a ‘quick fix’, economics and finances.
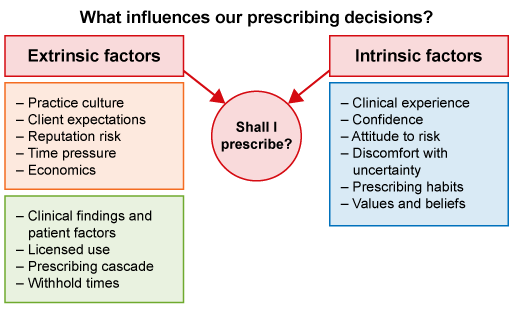
Extrinsic factors impacting on prescribing decisions are shown in Figure 7 and in particular include:
- The clinical findings and patient-related factors such as pregnancy status. Knowledge of the bacteria causing the illness and the physiological state of the animal may affect the activity of the antimicrobial.
- The registered uses of an antimicrobial, for example only using an antimicrobial registered to treat foot rot in cattle.
- Off-label use occurs when an antimicrobial is used to treat a condition that it is not registered to treat and/or in a species it is not registered to treat. Off-label use should only be done when there is no other registered alternative, the animal’s health and welfare is compromised, and follow the prescribing cascade.
- For some species, such as goats, there are few antimicrobials registered for use. In this case, off-label use of preparations registered in sheep used in accordance with other label directions is generally considered appropriate.
- The
withholding period (WHP) is a critical consideration in deciding what antimicrobial to use in food animals. The WHP is the time from the last treatment to when the animal can be slaughtered or milked (or eggs consumed), and residues are below the limit of food safety concern.- Long WHPs on some drugs (e.g. long-acting penicillin has a 30-day WHP) are more likely to be used when animals are not going to be slaughtered for a long time. In contrast, antimicrobials with short withhold periods (e.g. short-acting penicillin has a five-day WHP) are more likely to be used close to slaughter.
- When using drugs in an off-label manner, care should be taken to ensure animals are clear of residues before slaughter as the WHP is often extended.
- Client expectations and reputational risk can be critical factors related to why an antimicrobial may be given. Some studies have reported that veterinarians often feel pressured to prescribe antimicrobials and have a ‘just-in-case’ approach, fearing a risk to their business if they don’t meet client expectations.
- There is an economic incentive to prescribing and dispensing antimicrobials, and the income of veterinarians relies to some extent on the sale of drugs. This may influence prescribing decisions.
- In many countries, veterinarians must only prescribe antimicrobials that are registered for use in animals. However, this is not always possible or practical as there are few antimicrobials registered for use in certain animal species (e.g. fish) or for some uses of animals (e.g. dairy sheep). To strike a balance between legal prescribing requirements and the need to treat a wide range of conditions in animals, the laws in these countries permit a veterinarian to exercise their professional judgement to prescribe unregistered or off-label antimicrobials in certain circumstances. This is known as the ‘
prescribing cascade ’.
Intrinsic factors impacting on whether to give an antimicrobial or not are listed in Figure 7 and have a lot to do with behaviour and attitude to risk:
- Clinical experience helps with confidence in decision making, including deciding not to prescribe an antimicrobial. Prescribing can be driven by a lack of experience.
- Prescribing can be driven by the sense of discomfort or uncertainty with clinical evidence.
- Habits can be hard to break. Like doctors, veterinarians develop habits and patterns in prescribing antimicrobials. Another habit, driven in part by accessibility, reliability and economics, is to not request a diagnostic test which would enable an informed prescription.
Activity 8: Impactors on prescribing decisions
3.4.1 Guidelines for prescribing or giving antimicrobials to animals
You have probably heard the terms ‘appropriate’, ‘responsible’, ‘prudent’ or ‘judicious’ used in relation to antimicrobial use many times. Have you thought about what these terms mean to you?
Appropriate use means different things to different people. For example, suppose you are a veterinarian deciding to prescribe antimicrobials for a group of animals. You are likely to consider ‘appropriate use’ to mean ensuring that you are using the right antibiotic, at the right time, at the right dose, and for the right duration.
Someone who is not a veterinarian may think of appropriate use as ensuring that:
- antimicrobials are only prescribed by a veterinarian
- label directions and veterinary directions are followed
- the full course of antimicrobials is given
- HP-CIAs are not used as the
first line of defence or for preventative purposes with scientific justification.
A prescribing creed commonly used is the MINDME acronym:
- M – microbiology guides therapy where possible
- I – indications should be evidence-based
- N – narrowest spectrum possible
- D – dosage appropriate to site and type of infection
- M – minimise the duration of therapy
- E – ensure monotherapy if possible (one drug rather than combinations).
Determining appropriateness is not a straightforward task in food animals. It depends on several variables:
- access to diagnostic services
- training of veterinarians, farmers, and others in the supply chain
- availability and access to quality antimicrobials.
Fortunately, there are many resources to help ensure decisions about antimicrobial use are appropriate. For example, the OIE has detailed guidelines on responsible and prudent use of antimicrobials, which you can read on the OIE website when you have time.
Prescribing guidelines are freely available on many public websites for veterinarians. Prescribing guidelines help with choosing the most appropriate antimicrobial based on the animal species and the bacterial pathogen that is causing the disease. The best prescribing guidelines are those that are evidence-based, designed to be flexible, and specific to the local context.
Here are some prescribing guideline resources you can review in your own time:
https://www.ruma.org.uk/ antimicrobials/ guidelines/
3.5 The five principles (5 Rs) of antimicrobial stewardship in animal health
The 5 Rs are our roadmap for managing AMR in food animals (Figure 8). They are principles that guide us to use the right drug, in the right amount, at the right time, for the right reasons.
You can learn all about the 5 Rs of antimicrobial stewardship by watching this short Youtube video.
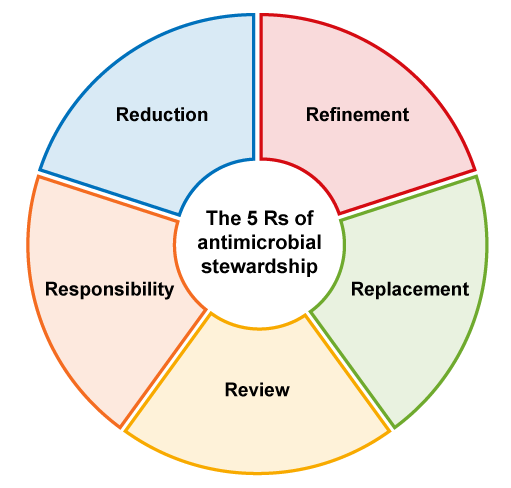
3.5.1 Responsibility
Everyone who uses antimicrobials is responsible for using them appropriately. This is especially true for veterinarians, who prescribe and dispense antimicrobials regularly, but it also includes farmers, workers, animal health advisors, and others involved in supplying or using the drugs in food animals.
3.5.2 Reduction
Antimicrobials are not always the only solution to fight infections. Reduction is about minimising the need for antimicrobials by being proactive and identifying innovative solutions to prevent disease. For example, improving husbandry practices like changing the ventilation system in a shed, or feeding a more nutritious diet, can help prevent disease. Less reliance on antimicrobials will improve economic returns and slow the development of resistant bacteria.
3.5.3 Refinement
When animals get sick, sometimes our best course of action is to use antimicrobials to treat the infection. This is where refinement comes into play. By determining the cause of the infection (using diagnostic tests), you can select the most appropriate antimicrobial to use. Continually evaluating diseases on farms and identifying the bacteria causing the illness allows veterinarians to refine antimicrobial use.
3.5.4 Replacement
Wherever possible, alternatives to antimicrobials should be used to treat infections. This is known as ‘replacement’. For example, vaccinations can often be used to prevent illness in farm animals, and the use of probiotics can improve gut health and the immune system.
3.5.5 Review
Review is the last step on the roadmap to antimicrobial stewardship. The decisions made today may not always be applicable in the future. Veterinarians should work with farmers to track and monitor changes in herd health on a regular basis. This includes tracking the number of antimicrobials used.
4 Complementary initiatives for antimicrobial stewardship in animals
Using antimicrobials to treat infections is just one part of animal health. Good husbandry, hygiene, biosecurity (Figure 9), and the use of non-antimicrobial treatment alternatives (e.g. vaccinations) reduce the disease burden on farms and the need for antimicrobials.
As animal production rapidly expands and intensifies in LMICs, education about alternative measures to prevent and treat infectious diseases suited to the local context is needed.

4.1 Biosecurity and hygiene
Biosecurity is one of the best strategies to prevent diseases on farms. At its heart, biosecurity aims to stop the introduction and spread of diseases in animals. Biosecurity is also an essential tool for AMS programmes; animals are less likely to become sick from infectious diseases and less likely to need antimicrobials. Biosecurity has the added benefit of improving animal welfare and productivity.
Biosecurity practices can be broken down into two types of actions.
- They prevent diseases being introduced from outside of the farm.
- They reduce infection spread within the farm by adopting cleaning and disinfection processes.
Table 3 lists the three elements of biosecurity on farms.
| Biosecurity type | Actions |
|---|---|
| External |
|
| Internal |
|
| Animal health |
|
Hygiene, or sanitation, refers to the cleaning and disinfection of people, equipment, animals and material either entering a farm or kept on the farm. Feeding, handling, administering medical treatments, vehicles and outside visitors are all possible contact points for transmitting diseases and pests. The biggest risk of introducing diseases occurs at the entry and exit points of the farm or buildings where the animals are housed.
To establish biosecurity on-farm, you need to have three zones:
- Dirty zone – this is where cleaning and disinfection occur before entry to the farm. Usually, this occurs at the farm gate or close to the perimeter of the farm.
- Buffer zone or line of separation – a transitional space between the dirty zone and where animals are housed. An additional level of cleaning and disinfection may be required here where all organic matter is removed prior to entering the clean zone.
- Clean zone (or controlled access zone) – this is where the animals are housed or kept.
You can learn more about on-farm biosecurity and sanitation methods on chicken farms by watching this YouTube video (using the subtitles) or visiting the Healthy Farms Healthy Agriculture website.
Activity 9: Biosecurity measures – what do they look like in practice?
Think about a typical poultry farm or fish farm in your country and draw a biosecurity map for the farm. Think about where you would place the various zones to ensure infectious diseases are not transmitted to the animals already kept at the farm.
Do you know of any farms in your area who already practise biosecurity and are they doing it well? Do you feel confident about talking to farmers about their biosecurity and where it can be improved?
Discussion
How did you go drawing your biosecurity map? If you watched the video and/or looked at the Healthy Farms Healthy Agriculture website, you would have been given the resources to draw a biosecurity map. Both sites give excellent tips and resources about engaging with farmers on good biosecurity practices.
4.2 Alternatives to antimicrobials
Alternatives to antibiotics can minimise antimicrobial use in animals. This is achieved by helping to prevent and control infectious diseases.
4.2.1 Vaccinations
Vaccinations are an effective way to prevent bacterial and viral diseases in people and animals. Making better use of existing vaccines and developing new vaccines are important measures to reduce the need for antimicrobials.
Vaccines work by training your immune system to recognise and respond to a pathogen. The immune response prevents the establishment of an infection, or it decreases the severity of the infection. If enough animals are vaccinated, you can achieve
Antibacterial vaccines prevent infections in animals that would otherwise require antimicrobial treatment, while
Vaccines present some challenges – notably to do with logistics. Many vaccines must be given by injection to individual animals, leading to increased labour costs and stress caused by handling. Also, many vaccines need to be kept cold or frozen. For vaccines to be more widely adopted in LMICs, they need to be accessible, cheap, easy to store and supported by government.
4.2.1.1 Alternatives to using AGPs
The bulk of antimicrobials used in animals is thought to be for growth promotion purposes, so using alternatives to AGPs is a critical priority for the agriculture sector.
There are a number of alternative products for growth promotion, including:
Probiotics – these are live microbes (yeast, fungi, bacteria) that improve the balance of bugs in the gut also have preventative health benefits. Good for all food animals.Prebiotics – these are organic compounds that are broken down by healthy bugs in the gut and selectively stimulate these bugs to grow.In-feed enzymes – these help animals break down and digest plant materials they otherwise cannot utilise effectively. Good for ruminants (cattle, sheep).Antimicrobial peptides – these are short molecules with antibacterial properties toxic to certain bacteria.
The Pew Charitable Trust has published a report on alternatives to antibiotics in animal agriculture (Pew Charitable Trust, 2017). If you wish you can find detailed information on the Pew Charitable Trust website.
Note, these alternative treatments need to be selected with care as some may be antagonistic or compete with each other.
4.3 Diagnostic stewardship in animal health
For a comprehensive review of laboratory methods for AMR look at: module E – Isolating and identifying bacteria; module F – Antimicrobial susceptibility testing; module G – Testing for mechanisms of resistance; and module H – Quality assurance and AMR surveillance.
Diagnostics are an important part of any health system. Without laboratory and pathology services, we would struggle to diagnose diseases, select the best treatment options, predict prognoses and monitor disease progression.
Diagnostic testing is a critical element of AMS. Diagnostic tests help to reduce antimicrobial use by:
- determining if a bacterium or another pathogen causes disease – if bacteria do not cause the disease, then the animal does not need antibiotics
- determining to which antimicrobials the bacteria are resistant or susceptible, allowing prescribers to select the most appropriate antimicrobial to use.
4.3.1 What is diagnostic stewardship?
You can find out about diagnostic stewardship in human health in the module V Diagnostic stewardship in clinical practice. Also, the WHO has published a guidance document on diagnostic stewardship, which you can read on the WHO website at some point in the future.
The WHO defines diagnostic stewardship as:
‘The coordinated guidance and interventions to improve appropriate use of microbiological diagnostics to guide therapeutic decisions. It should promote appropriate, timely diagnostic testing, including specimen collection, and pathogen identification and accurate, timely reporting of results to guide patient treatment.’
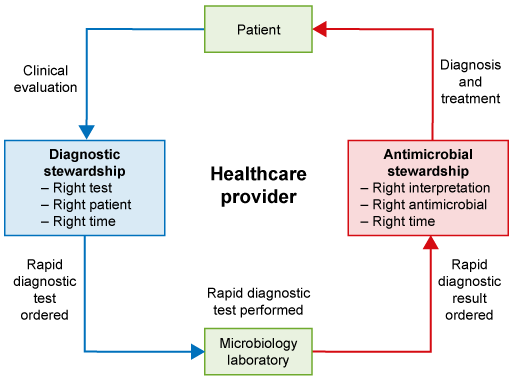
The difference between AMS and diagnostic stewardship can be seen in Figure 10. While AMS is about using the right drug at the right time at the right dose for the right duration, diagnostic stewardship is about obtaining the right test in the right patient to ensure the use of the right drug at the right time at the right dose for the right duration.
4.3.2 Rapid testing
At the heart of diagnostic stewardship is timeliness – specifically, the need to deliver timely laboratory results to guide efficient and effective treatments.
Microbiology laboratories typically take 4–5 days to provide phenotypic antimicrobial susceptibility testing (AST) results. It takes around 48–96 hours for isolation and identification and 48–72 hours for AST to be completed using traditional methods. It takes longer for test results for food animals because of the transport time for samples to reach the laboratory.
In contrast to conventional culture methods, rapid testing aims to provide results in minutes to hours.
A key concern of traditional testing is that antimicrobial treatment often commences before laboratory results are available. This is referred to as
Common technologies used for rapid testing include:
- antigen-lateral flow devices (
LFD ) SNAP tests (enzyme immunoassays for a range of diseases and chemicals)- real-time polymerase chain reaction (
qPCR ).
You can find out more about what these technologies involve here, as well as in the module E2 Isolating and Identifying bacteria (animal or human), at some time in the future.
There are many challenges in developing rapid tests that can perform as accurately and reliably as traditional methods. Many rapid tests are still in the development phase because of these issues and commercialisation costs.
In food animals, many of the rapid tests available or in development are designed to detect viruses (e.g. foot-and-mouth disease, rinderpest, high-pathogenic avian influenza). There are some laboratory-based rapid tests available for bacteria that can perform fast identification of bacteria and resistance genes or markers (e.g. PCR – see module E2) but they are usually only available in reference (and/or research) laboratories, access to which may pose transport and economic problems. However, ‘pen-side’ rapid tests for bacteria cannot yet replace the need for culture-based methods, and the equipment necessary to carry out the techniques listed above is relatively expensive, meaning it may have limited application in the animal health laboratories of many LMICs.
In practical terms, rapid test technologies that can be used in the field have the most potential in food animal medicine. These tests can be used as an initial screening test and assist with diagnostic decisions. For example, if the test confirms a viral pathogen, a decision may be made that antibacterials are unnecessary.
Rapid tests for use in the field for animal health should:
- be cheap to buy
- require minimal equipment
- be robust in field conditions (withstand dust, water)
- not require a power source
- be easy to use, with minimal instructions so farmers and farm workers can use them if needed
- need minimal sample preparation
- work at stable temperature (i.e. do not need refrigeration)
- produce rapid (
4.3.3 Selective reporting of antibiotic susceptibility test (AST) results
Did you know that studies have found that selective reporting of AST results positively influences prescribing practices and is associated with a decrease in AMU?
Selective reporting is when a microbiologist only reports the results for a limited number of antimicrobials instead of all the antimicrobials tested. Usually, the microbiologist will report antimicrobials that are:
- first line of defence
- relevant to the bacteria/species/site of infection
- narrow spectrum
- Not HP-CIAs (in animals).
The advice of microbiologists is critical to decisions about selecting the most appropriate antimicrobials – they are, after all, the experts in the field.
Selective reporting is well established in human medicine, but it is usually performed in individual hospitals or laboratories, based on local prescribing guidelines and drug availability, and there is no national or international consistency.
In veterinary medicine, the practice of selective reporting is not well established, but there is increasing recognition of the advantages of the practice in veterinary laboratories.
4.3.4 Challenges of veterinary laboratories in LMIC constraints
Compared to human medicine, there are many fewer veterinary microbiology laboratories, and they are often underutilised in food animal disease investigations. In LMICs, the shortage of veterinary microbiology laboratories, skilled staff, access to veterinary services, and the high cost of testing are key challenges. Also, many of the farming enterprises in LMICs tend to be small-scale, low-input systems in remote locations, making sampling challenging both in taking samples and transporting them to the laboratory.
The diagnostic challenges faced by LMICs are well recognised. Global organisations, including WHO, OIE, and
Innovative approaches to disrupt the traditional microbiology laboratory model are being explored, such as
Activity 10: Diagnostic stewardship
What do you understand by the term ‘diagnostic stewardship’ and how does it address the misuse of antimicrobials?
Discussion
Diagnostic stewardship essentially boils down to appropriate and timely use of diagnostic testing and reporting of results to guide therapeutic decisions. While it is borne out of human medicine, the principles can also be applied to veterinary medicine. Diagnostic stewardship is about obtaining the right test in the right patient to ensure the use of the right drug at the right time at the right dose for the right duration. By adopting these principles, antimicrobial use can be tailored to the infection and infection site to optimise use.
Of course, diagnostic stewardship can only be relevant to veterinary medicine when there are animal health or fish laboratories to support diagnostic testing. LMICs face particular challenges in fulfilling the principles of diagnostic stewardship, however global organisations, including WHO, OIE, and FAO, and international aid programmes such as the Fleming Fund (UK Aid) are partnering with a number of countries to build laboratory capacity and train staff in human and animal health laboratories.
5 International standards for prudent use of antimicrobials
In module L AMR surveillance in animals, you learned about the global One Health response to AMR which arose from the
Table 4 lists some of the more important global policy documents released since 2015. These policies have created the impetus for the One Health response to AMR. The policies listed in Table 4 are included for your interest or subsequent study and do not form part of the completion of this module.
| Date | Publication/action | Reference |
|---|---|---|
| 2015 | WHO Global Action Plan on Antimicrobial Resistance | (WHO, 2015a) |
| 2015 | WHO’s Global Antimicrobial Resistance Surveillance System ( | (WHO, 2015b) |
| 2016 | OIE Strategy on Antimicrobial Resistance and prudent use of antimicrobials | (OIE, 2016) |
| 2016 | FAO Action Plan on AMR (2016-2020) | (FAO, 2016) |
| 2016 | United Nations General Assembly adopted a declaration calling for all nations to respond to the global threat posed by AMR | (United Nations, 2016) |
| 2016 | First report: OIE Annual report on the use of antimicrobial agents in animals: A better understanding of the global situation. Subsequent annual reports released 2017, 2018, 2019 | (OIE, 2018b), (OIE, 2018) |
| 2017 | WHO guidelines on use of medically important antimicrobials in food-producing animals | (WHO, 2017b) |
| 2017 | WHO Guidance on Integrated Surveillance of antimicrobial resistance in foodborne bacteria | (WHO, 2017a) |
5.1 OIE Strategy on AMR and prudent use of antimicrobials
The OIE Strategy, released in 2016, aligns with recommendations in the WHO Global Action Plan. The OIE Strategy has four main objectives and several sub-objectives. Across all the objectives, there is an underlying theme of prudent use of antimicrobials (Table 5).
| Strategic objective | Antimicrobial stewardship goal |
|---|---|
| Improve awareness and understanding |
|
| Strengthen knowledge through surveillance and research |
|
| Support good governance and capacity building |
|
| Encourage implementation of international standards |
|
5.2 Global database on the use of antimicrobials in animals
The OIE has built a global database on the use of antimicrobial agents in animals. The survey aims to collect harmonised quantitative data on AMU in animals at a national level from all Member Countries. The OIE survey template used to collect data is designed to allow all countries to participate, regardless of whether a national data collection system currently exists. The survey is split into two sections, the first collect base-line information about a Member Country’s current situation concerning regulations etc., while the second section seeks quantitative data (Figure 11).
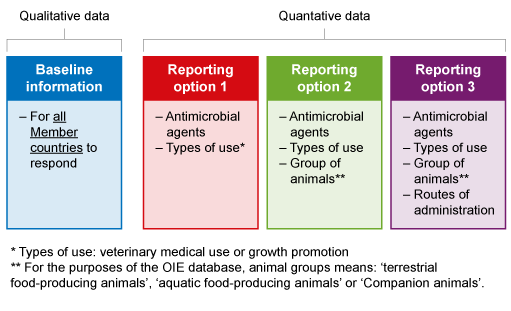
The first annual data collection on antimicrobial agents in animals in OIE Member Countries commenced in 2015, with the most recent report published in 2020. Each year, more member countries participate in the survey. If you would like to read more about the reports, you can find them on the OIE website.
5.3 OIE international standards for prudent use of antimicrobials in animals
The OIE has also developed international standards for using antimicrobials. The standards are published in Chapter 6.10 of the Terrestrial Animal Health Code (OIE, 2018a)
Chapter 6.10 defines various stakeholders' roles and responsibilities, including regulators (Competent Authority), veterinary pharmaceutical industry, animal feed manufacturers and farmers. It has developed standards for antimicrobials from the manufacturer through to the farmer.
To read more about the standards, visit the OIE website when you have time.
5.4 National action plans
In 2015, the WHO Assembly adopted the global action plan (
The WHO also compiled an electronic library of many of the NAPs which you can search on the WHO website when you have time.
6 Advocating for antimicrobial stewardship in animal health
The adage that ‘prevention is better than cure’ holds firm for human and animal health. The need for an integrated One Health approach to AMS and AMR cannot be overstated. All stakeholders, from government agencies, veterinary pharmaceutical industry, veterinarians, paraveterinarians and farmers, need to work collaboratively to promote the responsible use of antimicrobials.
Now that you have worked through some of the key issues related to AMS, it is time to put your learning into action.
There are numerous resources available to you to advocate AMS in animal health. Here are just a few for you to visit when you have the time:
- OIE, FAO, and WHO have developed a Trello board of communication materials you can use, share and spread. You can visit the Trello website.
- The ReAct Toolbox is a repository of resources on AMR and stewardship.
- Take the pledge to be an antibiotic guardian. As of February 2021, over 1.3 million people have pledged to be antibiotic guardians.
- The National Centre for Antimicrobial Stewardship in Australia has many resources focused on antimicrobial stewardship in animals, including a template for your antimicrobial stewardship plan, prescribing guidelines for food animals, and posters to download.
Activity 11: Applying your experience and planning how you can influence AMS
Here are some activities to get you started on your mission. Perhaps, you could start now and revisit when you’ve studied other modules and gained more insight:
Veterinarian and veterinary paraprofessionals:
If you are a veterinarian or veterinary paraprofessional, take some time to outline your antimicrobial stewardship plan relevant to your local context.
Here are some resources to help your get started:
- The National Centre for Antimicrobial Stewardship and the University of Melbourne have a template available for veterinarians. The template can be adapted to suit your local situation. You can download and modify the ASP template.
- The American Veterinary Medicine Association (AVMA) has an antimicrobial stewardship checklist which you can download as a pdf.
- The University of Minnesota has recently published the Handbook on Antimicrobial Stewardship in Companion Animal Veterinary Settings. It contains helpful summaries and tips when developing an antimicrobial stewardship plan. While it focuses on companion animals, you may find there are elements of the handbook that are adaptable to your local context. You can review the handbook in pdf format.
In writing out your plan, think of the following points:
- What is the purpose of your plan? You may be a private veterinarian working directly with poultry farmers, pig producers, cattle or fish farms in your district. Or maybe you work for the Government, and you visit farms to promote animal health and look at disease outbreaks. In these scenarios, the overall purpose of the plan may be the same, i.e. the responsible and judicious use of antimicrobials, but the interactions with farmers and subsequent approach to prescribing may be different.
- Interventions in the context of antimicrobial stewardship mean what actions you will take to protect antimicrobials. This may be committing to:
- following prescribing guidelines
- not prescribing or using HP-CIA antimicrobials in food animals
- setting up a traffic light system (green-orange-red) for antimicrobials based on first, second and last line of defence drugs
- using diagnostic tests to confirm infections and inform antimicrobial selection
monitoring AMU on your client’s farms- using alternatives to antimicrobials where possible.
- What role will you play in educating yourself, your staff/team members and your clients about antimicrobial stewardship? A range of activities could be identified here, such as ensuring you and staff participating in continuing education (e.g. courses, conferences, reading) and regularly discussing stewardship, good husbandry and biosecurity practices with your clients.
7 Conclusion
In this module, you have learned about AMS in animal health. You have reviewed issues related to the use of antimicrobials for therapeutic and non-therapeutic purposes, the role antimicrobials play in animal welfare. We also looked at the principles of biosecurity and diagnostic stewardship and how they apply to veterinary medicine.
After completing this module, you should now be able to:
- define the five principles of AMS in animal health
- describe how intrinsic and extrinsic factors drive prescribing behaviour
- list and explain the different therapeutic and non-therapeutic uses of antimicrobial agents in food animal production
- identify the relationship between AMS and animal welfare
- list antimicrobial agents rated as critically important for people that are commonly used in food animal production.
Activity 12: Reflecting on your progress
Do you remember at the beginning of this module you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas? Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas.
Now that you have completed this module, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself.
8 End-of-module quiz
Well done – you have reached the end of this module and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
9 Resources
9.1 Online antimicrobial stewardship courses
- Future Learn course Antimicrobial stewardship in veterinary practice – self-paced.
- AMR Vet Collective Online Vet AMS course, self-paced, hosted by the University of Sydney and Charles Sturt University. The website also hosts a suite of educational materials, including prescribing guidelines for poultry, dairy cattle, beef cattle, pigs and sheep.
- OpenWHO online course on competency-based approach to AMS.
- Antimicrobial Resistance Learning Site (AMRLS), hosted by the University of Minnesota, has a suite of educational materials to teach and promote the prudent use of antimicrobial agents in veterinary practice.
- British Society for Antimicrobial Chemotherapy (BSAC) Infection Learning Hub – open-access global learning on AMR and antimicrobial stewardship.
9.2 Other useful resources for planning an antimicrobial stewardship plan or education activities
- OIE AMS website – a website that provides stakeholders with AMS communication resources – designed for vets, farmers, policymakers, pharmaceutical industry, stock feed manufacturers.
- Antimicrobial stewardship guidelines for the Australian cattle feedlot industry. Published by Meat and Livestock Australia. Includes practical information for veterinarians and farmers to develop AMS plans specific to each farm. Can be applicable to sector 1 and 2 farms in LMICs.
- Farmed Animal Stewardship Initiative (FAAST) – veterinary AMS resources for farmers and veterinarians, supported by the Canadian Government, veterinary associations and industry. Videos, podcasts and resources available.
- The FAO has recently published the Handbook on responsible use of antibiotics in livestock production for animal health workers in Viet Nam. You can review it on the FAO website.
- Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit – while focused on human health, there is applicability to antimicrobial stewardship in food animals in LMICs. You can access the document via the WHO website.
References
Acknowledgements
This free course was collaboratively written by Skye Badger and Judith Taylor, and reviewed by Lucy Brunton, Claire Gordon, Natalie Moyen, Peter Taylor and Hilary MacQueen.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Images
Module image: magic pictures/Shutterstock.
Figure 1: OIE (2017). Reproduced with permission.
Figure 2: cheechew/iStock/Getty Images Plus.
Figure 3: ‘Veterinary antimicrobial stewardship: more than just a prescription’, FAAST, https://www.amstewardship.ca/ video/ veterinary-antimicrobial-stewardship-more-than-just-a-prescription/.
Figure 4 (pills): freestocks/Unsplash.
Figure 5: Van Boeckel et al. (2015).
Figures 6 and 7: ‘Why does antimicrobial stewardship matter and who is responsible?’, British Society for Antimicrobial Chemotherapy, https://www.futurelearn.com/ info/ courses/ antimicrobial-stewardship-in-veterinary-practice/ 0/ steps/ 65398.
Figure 8: adapted from ‘Antimicrobial stewardship for a sustainable tomorrow’, FAAST, https://www.amstewardship.ca/ video/ antimicrobial-stewardship-for-a-sustainable-tomorrow/.
Figure 9: Matthew Maaskant/FreeImages.com.
Figure 10: Messacar et al. (2017).
Figure 11: OIE (2019) ‘OIE Global Conference on Aquatic Animal Health’, https://youtu.be/ 77s3sfZbrpg?t=23370. Reproduced with permission.
Tables
Table 1: adapted from OIE (2019). Reproduced with permission.
Table 2: Scott et al. (2019). This file is licensed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) licence, https://creativecommons.org/ licenses/ by-nc/ 4.0/.
Table 3: based on MLA (2018).
Table 5: adapted from OIE (2016). Reproduced with permission.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.