Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 23 November 2025, 10:26 AM
Antimicrobial stewardship in clinical practice
Introduction
In healthcare, the four components of AMS are:
- structures to oversee stewardship
- facility-level interventions such as local antimicrobial prescribing guidelines
- patient-level interventions based on clinical and laboratory evidence (see the Diagnostic stewardship in clinical practice course)
- monitoring and evaluating
antimicrobial use (AMU) .
Education and training are also essential for clinicians to understand their role in the appropriate use of antimicrobials.
After completing this course, you will be able to:
- understand the principles of an AMS programme
- understand how to approach setting up an AMS committee
- indicate the place of the laboratory in the AMS process
- develop local prescribing guidelines
- describe AMS strategies such as prospective audit and feedback
- understand quantitative and qualitative ways to assess antimicrobial consumption and use
- appreciate the importance of understanding the pharmacokinetics and pharmacodynamics of antimicrobials in determining the optimum treatment strategy
- appreciate that AMS can be introduced gradually, and that initial emphasis on easier-to-achieve targets can lead to significant improvements
- refer to treatment guidelines and resources (such as WHO courses)
- describe the costs and benefits (cost-effectiveness) of implementing AMS.
In order to achieve your digital badge and Statement of Participation for this course, you must:
- click on every page of the course
- pass the end-of-course quiz
- complete the course satisfaction survey.
The quiz allows up to three attempts at each question. A passing grade is 50% or more.
When you have successfully achieved the completion criteria listed above you will receive an email notification that your badge and Statement of Participation have been awarded. (Please note that it can take up to 24 hours for these to be issued.)
Activity 1: Assessing your skills and knowledge
Before you begin this course, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Do not worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these courses.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 The concept of AMS
Activity 2: Initial reflections
Think about your healthcare facility and your role in it.
- Is there a general awareness of the need for stewardship of antimicrobials?
- Is there a structure in your facility such as a stewardship committee? Or is stewardship part of the role of other committees, such as the drugs and therapeutics committee?
- If you have a stewardship committee, do you feel that it communicates effectively? Does it educate individuals to make appropriate decisions on AMU?
- Are you aware of audits or surveys such as point prevalence surveys (PPSs) to monitor antimicrobial prescribing practice?
Note down your thoughts in the space below.
Discussion
You might have thought that there is some awareness of AMS in your facility but that clinicians are still making individual prescribing decisions without enough guidance, or that you are not receiving updated guidance based on monitoring of AMU.
AMU in human and veterinary medicine is one of the main drivers of
AMS may not be prioritised in resource-poor settings, but it is estimated that in selected low- and middle-income countries (LMICs), the proportion of resistant infections ranges from 40 to 60% compared to an average of 17% for high-income countries belonging to the Organisation for Economic Co-operation and Development (OECD; WHO, 2019b). This demonstrates the need for introducing or improving stewardship in all healthcare settings, and this course will address some key components in the process.
Stewardship measures aimed at optimising AMU decrease the development of AMR while reducing associated costs: this has been shown in African countries and provides encouragement for LMICs to develop AMS (Akpan, 2020). Healthcare facilities are at major risk of developing problems with AMR because they are major consumers of antimicrobial agents, and so they benefit enormously from AMS.
However, it is important to understand that AMS programmes are not unique to healthcare facilities and community practice, although this will be the focus of this course. AMS is also relevant to AMU in domestic, wild and food animals, as well as agricultural AMU on crop plants.
Stewardship in this wider context will benefit individuals being treated for infection, and reduce potential AMR in pathogens that cause animal and plant diseases. It will also reduce the potential spread of AMR in the environment through transmission of resistant microbes and antimicrobial genes, as well as antimicrobials and their metabolites excreted into soil or entering via wastewater (see the Introducing a One Health approach to AMR course).
If your country has a National Action Plan (NAP), it is likely to include recommendations for antimicrobial stewardship and optimising the use of antimicrobials. This is covered extensively in the UK’s most recent NAP, covering the period 2024–29 (UK Government, 2024); see particularly ‘Theme 2: Optimising the use of antimicrobials’. How does this compare with your own country’s provisions?
1.1 AMS in the clinical setting
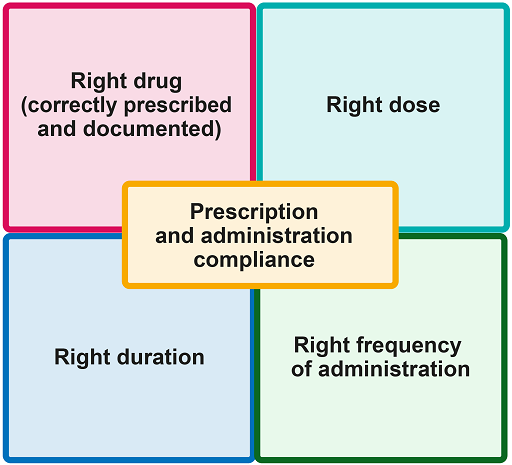
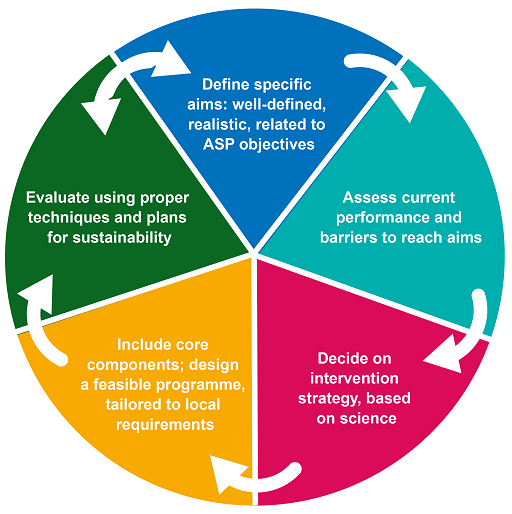
AMS in the clinical setting refers to responsible AMU by healthcare professionals; and, more specifically, to selecting the most appropriate antimicrobial, duration, dose and route of administration for a given patient with a demonstrated or suspected infection. This is summarised in Figure 1.
The decision to recommend particular antimicrobials for specific clinical conditions depends on:
- AMR surveillance data produced at the local level as a result of good diagnostic stewardship (see the Diagnostic stewardship in clinical practice course)
- implementing surveillance programmes at national, regional and facility levels.
Establishing surveillance programmes for AMR is one of the objectives of a national action plan, and should be directed by action points once this programme is endorsed. Surveillance for AMR provides data on the levels of susceptibility or resistance to tested antimicrobials. This data can be analysed to formulate evidence-based recommendations that inform and guide clinicians, policy-makers and others on how to use antimicrobials.
For example, based on this data, empirical treatment guidelines can be put in place at different healthcare levels. It is important to understand that levels of resistance of specific organisms to specific antimicrobials may change over time, and ongoing surveillance is critical to provide information on trends and current resistance patterns. Periodic reporting of AMR data is essential to allow the provision of up-to-date recommendations for empirical treatment.
Similarly, surveillance data is also necessary to inform the choice of directed therapy when the causative organism and susceptibilities are known: if an organism is susceptible to a number of effective drugs, clinicians may choose to prescribe an antimicrobial less likely to be associated with particular resistance problems in their unit.
2 How to establish AMS structures at different levels
National-level structures are required for effective AMS. Governments should develop their own national action plan for AMR, ideally as part of a wider One Health governance structure that engages multiple government sectors to establish an AMS approach that is aligned across human, animal and environmental sectors.
AMS can be overseen by ministerial committees, multi-sectoral co-ordinating committees, and technical working groups, or similar structures appropriate to the national situation. The role of these government structures is to implement policies, develop guidelines and allocate funds. (For more information on national structures, see the Introducing a One Health approach to AMR course.)
In the human health sector, AMS is one of ‘three pillars’ of an integrated approach to combat AMR as part of an overall goal to strengthen health systems (WHO, 2019a). These pillars, which are also relevant in veterinary medicine, are:
- AMS
infection prevention and control (IPC) - medicine and patient safety.
These pillars should be applied alongside AMU surveillance and monitoring, together with adequate provision of quality-assured medicines at a national level. Education and training the relevant professionals, as well as raising awareness and understanding of the issues among the general public, are essential components of this programmatic approach (WHO, 2019a, 2021).
2.1 Where to start: hospital facility-level AMS structures
AMS can be introduced or progressed in individual healthcare facilities by building on existing capabilities and resources using a stepwise approach. The starting point is to assess the current situation. Usually healthcare settings have pharmaceutical and therapeutic committees – often referred to as drugs and therapeutics committees (DTCs) – that oversee the use of all drugs used at the institution. These already existing committees could initiate the AMS programme.
The WHO has produced a toolkit, Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries (WHO, 2019) as a practical guide for setting up AMS structures at national, regional and healthcare facility levels. The elements of each programme are stratified into core and advanced (which require more facilities) and the exact nature of each programme will depend on the situation and the resources available.
The first step is to assess any AMS strategies already in place, and consider additional strategies that can be easily implemented. Areas for assessment could include:
- the availability of data on antimicrobial consumption
- whether prescription audits are routinely performed
- whether there is appropriate training for healthcare staff available at the facility.
The assessment can then be used to allocate human and financial resources to AMS and set up sustainable governance structures. These should be multi-disciplinary but may depend on existing structures and resources: for example, the AMS committee could be stand-alone or embedded in another existing committee such as the DTC.
Sample terms of reference for AMS structures, including for a hospital-level AMS committee, are set out in the WHO toolkit (WHO, 2019).
The membership of such a committee, which is set out in Annex II of the WHO toolkit in more detail, is likely to include:
- the administrator of the healthcare facility (chair)
- a director of medical services (vice chair)
- a clinical lead, physician or microbiologist
- a physician or pharmacist to act as secretary
- directors of other departments
- a patient safety and clinical manager
- representatives from nursing, pharmacology, microbiology and the different wards
- representatives from other relevant committees (e.g. DTCs) if the AMS committee is not embedded in one of these
- other members co-opted as necessary.
Regardless of whether or not the AMS committee is separate or a sub-committee of the larger DTC, there should be collaboration between the AMS committee and those overseeing IPC programmes and
The AMS committee should produce regular activity reports on the implementation of the AMS programme, both for their own healthcare facility management and for dissemination to regional/national structures. These reports should include data on AMU, resistance patterns for key pathogens and interventions implemented by the AMS team. The AMS committee is also responsible for ensuring that up-to-date standard treatment guidelines and ongoing training are available to all staff.
2.2 AMS in primary healthcare systems
This course focuses primarily on AMS in hospitals, but AMS is also very important in primary healthcare (PHC) systems, such as a family doctor, pharmacist or local clinic. The most important elements of AMS in PHC are as follows:
- Commitment: A commitment from all healthcare team members to prescribe antimicrobials appropriately and engage in AMS. This approach is critical for improving antimicrobial prescribing.
- Policy and practice: Use evidence-based diagnostic criteria and treatment recommendations for patient management.
- Tracking and reporting: This is called ‘audit and feedback’. It can guide changes in practice and can be used to assess progress in improving antimicrobial prescribing.
- Education and expertise: Education on appropriate AMU can involve patients and clinicians. Educating patients and family can improve health literacy and enhance efforts to improve AMU. Education for clinicians and clinic staff can reinforce appropriate antimicrobial prescribing and improve the quality of care.
In high-income countries AMS in PHC settings may use an integrated database system, which includes information technology support to pharmacists for processing prescriptions, and preparing and dispensing medication. This approach is recommended based on the evidence from a US study (Sanchez et al., 2016).
However, this integrated technology is unlikely to be available in LMICs, so a stepwise approach could begin by setting up a regional or local AMS committee, comprising representatives from medical, pharmacy and laboratory staff. Because of the weakness of many LMIC PHC systems, improved funding and commitment at all levels, and education about AMR, are key to improving AMS.
Educating the general population about AMR and correct AMU is important at a national or regional level, because general awareness of this problem is poor: patients’ demands for antimicrobials often result in inappropriate prescriptions being made, and over-the-counter purchases of antimicrobials is also common. Healthcare workers should also be provided with specific information and training to improve their awareness, which can be poor in situations where they are under-resourced and under pressure.
People in geographically remote locations, or in areas where there are too few healthcare workers, may consult untrained health assistants. Therefore, education about the issues is essential: radio, television and other media may be used most effectively. Approaches such as healthcare workers explaining AMR and correct AMU to the patient while providing the prescription can also improve general awareness (Rijal et al., 2021).
Activity 3: Improving AMS in your setting
If you work in a hospital or PHC setting, think about:
- how you might improve communication and involvement of relevant professionals
- other measures you could take to improve AMS.
Discussion
Some of the things that you will have thought of will be specific to your setting. You may also have considered the following:
- Enhancing the involvement of pharmacists by regular meetings or teleconferences, and developing integrated databases if the computer facilities are available.
- Ensuring timely updates on local AMR susceptibility data by enhancing the communication between the laboratory or clinical microbiologist, pharmacists, and prescribers.
- Implementing a review of the local facility prescribing practice based on local known resistance patterns, carried out by representative pharmacists, medical and laboratory staff.
- Using regular audit and feedback to provide up-to-date prescribing guidelines, and disseminating these effectively (either electronically or as posters).
- Regular in-service training for medical staff, with input from clinical microbiology and pharmacy staff.
- Using posters and media to improve the awareness of patients and local people of the importance of appropriate AMU.
- Using leaflets to explain AMR and correct AMU to patients at the point of prescription.
- Arranging training on AMR for all staff at your PHC facility, either in person or using online courses.
- Nominating one person at your PHC facility to undertake more extensive training relevant to AMS; they can become a local expert who can educate others.
3 Behavioural change
A change in the outlook, understanding and behaviour about AMU is essential in both healthcare facilities and society in general. The aims of behavioural change are to:
- increase the awareness and understanding of AMR, and responsible AMU
- dispel common myths and misconceptions among the public about AMU
- help the public to understand what AMR means, and how it affects them and their families
- demonstrate examples of inappropriate AMU, such as for viral infections.
Effective behavioural change requires individuals at every level of healthcare to be involved and understand their role, details of which should be included in relevant policies, job descriptions, and updated procedures, equipment and staffing. At the facility level, educational material in the form of posters and leaflets can be targeted at patients; at a national level, the wider public should be engaged with media campaigns to promote awareness.
There is always going to be resistance to change within organisations and among individuals. However, you can encourage acceptance and ‘sell’ the change idea within the healthcare facility by:
- explaining the benefits beyond helping patients (what’s in it for me?)
- providing credible evidence – storytelling (the story of me, the story of we, the story of us)
- creating a culture of involvement (design to implementation, reporting success, regularly informing participants)
- enabling your clinical leaders
- enabling your senior leaders.
In any community, there are people whose behaviours enable them to find better solutions to a problem than their peers. An AMS committee may identify these individuals and engage them in a specific role as champions of AMS, to play a central part in cultural change.
Behavioural change can also be promoted by antibiotic awareness campaigns such as World Antimicrobial Awareness Week, and other targeted campaigns at national or local community level (WHO, n.d. 2). An example of a national campaign is the Antibiotic Guardian initiative, set up in 2014 by the UK Health Security Agency (UKHSA). This encourages all individuals – whether ‘health or social care professionals’, ‘students, educators or scientists’ or simply members of the public – to select a suitable pledge to use (or promote the use of) antibiotics wisely that is appropriate for their particular role. Families and educators are further directed to the e-Bug site, which provides educational games and resources for teaching children and young people of 3–16 about antibiotics and their responsible use.
4 Local guidelines and surveillance for antimicrobial prescribing
The use and resistance profiles of antimicrobials will vary according to the location and setting, so it is necessary for an AMS committee to draw up local guidelines for the appropriate choice of antimicrobials. If local healthcare facilities do not have available antimicrobial susceptibility testing data, they should use national AMR surveillance data. In order to guide this process, the WHO has classified antimicrobials using the AWaRe system.
Activity 4: The WHO’s AWaRe classification
5 Interventions
AMS interventions describe regulations and guidelines at facilities and clinical decision-making at patient level. The following sections provide more detail on facility and patient-level AMS strategies.
You might also like to look at an AMS toolkit developed for use by inpatient facilities in England, ‘Start smart, then focus’, as an example of a strategy that is proving useful in a high-income country. This was last updated in 2023.
5.1 Facility-level interventions
Antibiograms
Facility-level interventions are guided by data collected on the antimicrobial susceptibility profile of bacterial isolates in a hospital. This is usually provided in the form of a hospital
A typical hospital antibiogram is shown in Table 1. The first rows list the bacteria, separating them into Gram-positive and Gram-negative. The row below that shows the number of patients in the facility who had the organism and were included in the antibiogram; only the first isolate from each patient is included. Antimicrobials tested and the organisms’ susceptibilities are listed in the remaining rows.
| Hospital antibiogram annual report on percentages of susceptibility rates to selected antibiotics | Gram-negative | Gram-positive | |||||
|---|---|---|---|---|---|---|---|
| Ec | Kp | Pa | MSSA | MRSA | Sp | Espp. | |
| Total isolates | 2215 | 532 | 446 | 509 | 312 | 47 | 295 |
| Penicillin | 97 | ||||||
| Ampicillin | 41 | 87 | |||||
| Ampicillin/sulbactam | 54 | 72 | |||||
| Piperacillin/tazobactam | 94 | 95 | 92 | ||||
| Ceftriaxone | 85 | 79 | 97 | ||||
| Cefepime | 88 | 79 | 91 | ||||
| Meropenem | 100 | 99 | 87 | ||||
| Ciprofloxacin | 77 | 75 | 41 | ||||
| Oxacillin | 100 | 0 | |||||
| CTX | 76 | 83 | 96 | 94 | |||
| Nitrofurantoin | 97 | 35 | 99 | ||||
| Gentamicin | 91 | 93 | 90 | ||||
| Vancomycin | 89 | ||||||
Footnotes
(Ec = E. coli; Kp = Klebsiella pneumoniae; Pa = Pseudomonas aeruginosa; Sp = Streptococcus pneumoniae; Espp. = Enterococcus spp.)Activity 5: Looking at an antibiogram
Read the antibiogram in Table 1 and answer the following questions:
- How many people had Pseudomonas aeruginosa infections?
- Of these, what percentage of isolates were susceptible to ciprofloxacin?
- How would you use the antibiogram to select an antimicrobial for empirical treatment of Pseudomonas aeruginosa in this hospital?
Answer
- Pseudomonas aeruginosa was isolated in 446 people. It was tested for susceptibility to a number of antibiotics.
- Of the Pseudomonas aeruginosa cultures tested against ciprofloxacin, only 41 per cent were susceptible to the antibiotic.
- The hospital antibiogram shows a high rate of resistance to ciprofloxacin, so this is not an appropriate choice for empirical therapy; your empirical antibiotic choice should consider antimicrobials with susceptibility higher than 75%. In this example you might choose cefepime, meropenem, gentamicin or piperacillin/tazobactam, because Pseudomonas aeruginosa isolates from this hospital in the last year have been at least 87 per cent susceptible to these antimicrobials.
A short article on the FutureLearn platform includes more information about antibiograms, including how to create them.
Antimicrobial timeouts
An
Antimicrobial timeouts can be automatically incorporated in electronic prescribing systems; but in their absence, it may be most convenient for the pharmacy to alert clinicians of the need for reassessment, usually after 48–72 hours. If the hospital is also carrying out prospective audit and feedback, the individuals collecting the data can also take responsibility for monitoring.
Pre-prescription authorisation
One effective way of reducing costs and AMU on the WHO Watch or Reserve lists is pre-prescription authorisation for the use of these drugs.
A clinician should complete an authorisation form for any agent on Watch or Reserve lists, with all the necessary information for the signature of a designated expert in AMR (such as an infection pharmacist, clinical microbiologist or infectious diseases physician). The drug cannot be dispensed unless authorised by one of these experts, although some facilities will allow a first dose to be given without authorisation for patients presenting with sepsis who require immediate treatment.
Pharmacy departments are custodians of these forms, which, once collected, can be analysed for audits or for giving institutional feedback at facility level. The designated expert may suggest alternative antimicrobials, if appropriate.
Prospective audit and feedback
Another AMS strategy is prospective audit and feedback, where the clinician prescribes antimicrobials as usual but the prescription is later reviewed case-by-case by a pharmacist or infectious disease physician, who provides feedback and discusses it with the prescriber.
This has the benefit of allowing the clinician more autonomy, while simultaneously enhancing their understanding. An example in South Africa used pharmacist-driven audit and feedback (Brink et al., 2016), as discussed in Video 1.
Transcript: Video 1 Using existing resources to embed an AMS programme (FutureLearn, n.d. 1).
Hello, my name is Adrian Brink. I’m a clinical microbiologist from Johannesburg, and I’d like to share with you a specific antimicrobial stewardship programme that we’ve been involved in.
In South Africa, we don’t have enough infectious disease resources, neither microbiologists in all hospitals. We also don’t have IV pharms or clinical pharmacists in every hospital, so we had to use existing resources to embed a initial basic stewardship programme – a sustainable one – in all of the hospitals that participated in this programme.
So we chose a prospective audit pharmacist – a non-specialised pharmacist – to have an audit and feedback strategy, to implement across 47 rural and urban hospitals. The interventions that the pharmacist chose or that we chose for the pharmacist were so-called ‘low-hanging fruit’, implying that they are easy, obtainable interventions without IV resources. They were, for example, duration of therapy longer than seven days, duration of therapy longer than 14 days, redundant cover – many doctors don’t know overlapping spectra between Gram-negative and Gram-positive antibiotics. We also did with them an intervention to make sure that cultures were taken prior to empirical therapy, for example.
The target was these five low-hanging fruit, and every pharmacist then were allocated stewardship time from their daily activities to go to the wards and ICUs, and to measure patients more than seven days of therapy, 14 days, et cetera, intervene, and discuss that with the doctor to reduce overall consumption, which was the aim of the study.
The model that we used for improvement amongst the non-IV forms was the Breakthrough Series Collaborative, which you’re going to learn about later. It involved six-weekly or two-monthly teleconferences with all the pharmacists in the so-called PDSA cycles, which you’re also going to learn of. Overall, the five interventions over a two-year period led to a 12.5% reduction in overall consumption in these 47 hospitals.
-
What were the key aspects of the study in Video 1?
-
You may have noted that:
- the interventions chosen were straightforward and did not require new resources
- time was allocated to pharmacists to visit wards and talk to clinicians
- measurements included use of an antimicrobial for more than seven days, more than ten days and concurrent use of four different antimicrobials
- there were bi-monthly teleconferences of pharmacists from the different hospitals and rounds of education.
Choosing the best strategy
Choosing the appropriate strategy for your healthcare facility – either pre-prescription authorisation or prospective audit and feedback – is something that must be discussed by the AMS committee and can be adapted. More than one strategy can be adopted, but it is usually best to introduce them in a step-wise manner to improve acceptance and compliance.
The following examples illustrate how the approaches differ:
- Prescription of carbapenems:
- Pre-prescription authorisation: Restrict the use of all carbapenem antibiotics until authorisation by the pharmacist, microbiologist or similar, according to local guidelines.
- Prospective audit and feedback: Regular bedside ward rounds by the AMS team to review prescription charts for carbapenems and other reserve antibiotics.
- Prescription of individual drugs:
- Pre-prescription authorisation: Require clear diagnosis before release of ceftriaxone by pharmacist.
- Prospective audit and feedback: Regular chart reviews and feedback by the pharmacist to check that doses are correct, and the indication and duration are clearly recorded. Courses of antimicrobials lasting more than seven days, without a clear indication for prolonged treatment, are discussed with the prescribing clinician.
5.2 Patient-level interventions
Antimicrobial prescription needs to be monitored at the level of each individual patient for the benefit of that patient, and to reduce the selection pressure that leads to the development of resistance.
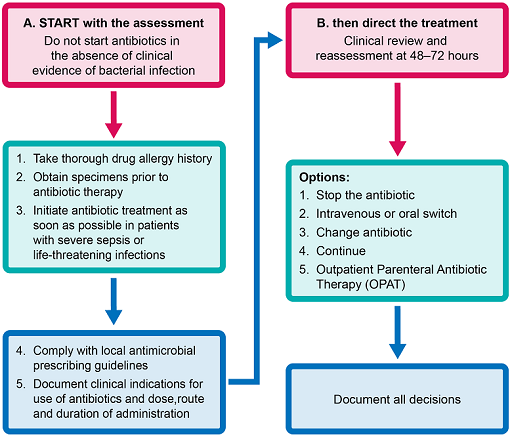
Figure 3 shows the sequence of decisions that may be needed at the level of patient treatment.
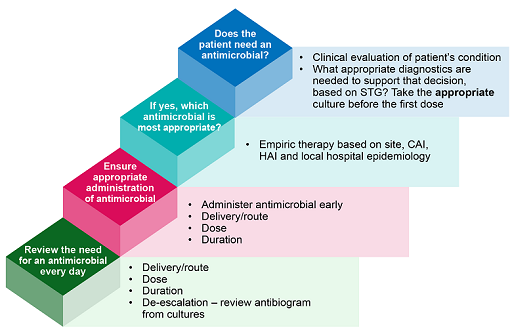
Choosing the correct drug for empirical treatment depends on clinical assessment and the local antimicrobial susceptibility data. Once culture and antimicrobial susceptibility test results are available for an individual patient, the antimicrobial with the narrowest spectrum of activity should be used as a treatment. If no organism is cultured from the samples, clinical reassessment determines the course of action.
Dosing and dose adjustment
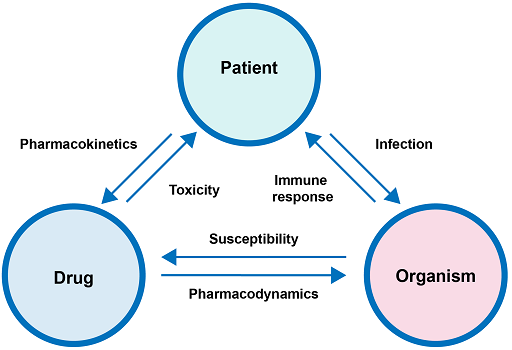
In order to understand the appropriate dose of a drug needed for clinical efficacy in a patient, it is necessary to understand pharmacokinetics and pharmacodynamics parameters (Figure 4).
The pharmacology of antimicrobial therapy can be divided into two distinct components:
- Pharmacokinetics: What the body does to the drug. Pharmacokinetic parameters include absorption, distribution, metabolism and elimination.
- Pharmacodynamics: The relationship between the serum concentration and the pharmacological (mechanism of action) and toxicological effects of drugs.
It is not necessary to understand the details of pharmacokinetics, pharmacodynamics and the resulting dosing schedules to implement AMS. However, the following sections on these aspects have been included in this course for your information, because they explain why different drugs must be administered according to particular schedules for efficacy, and to avoid toxicity to the patient.
Guidance on correct dosing must be readily available to all clinicians at your facility (such as a family doctor, pharmacist, local clinic or hospital). This guidance must be followed systematically, also taking into consideration individual characteristics of the patient such as allergies or renal problems.
Note that you will not be tested on the following sections in the end-of-course quiz.
Pharmacokinetics
Note that you will not be tested on this section in the end-of-course quiz.
AMU and the design of effective dosage regimens are based on the relationship between the administered dose of a drug, the resulting drug concentrations in various body fluids and tissues, and the intensity of pharmacologic effects caused by these concentrations. The journey of the antimicrobial through the body occurs in steps: absorption, distribution, metabolism and elimination (ADME). This is shown in Figure 5 and described in more detail below.
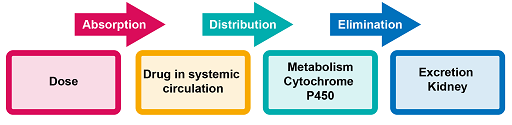
Absorption
Most antimicrobials are administered orally; various factors affect their ability to achieve the expected activity. Absorption is complex and involves several possible mechanisms. Passive diffusion has been identified as the predominant mechanism of gastrointestinal absorption for most drugs. Physiological characteristics such as gastric emptying time and pH conditions throughout the gastrointestinal tract also affect oral absorption. The presence of food, other drugs and certain digestive disorders may further change the rate and extent of absorption.
Distribution
Once a drug has entered the vascular system, it becomes distributed throughout the various tissues and body fluids. However, most drugs do not distribute uniformly throughout the body: their distribution is based on tissue-specific differences in rate and extent of drug uptake, including blood flow and the delivery of drug to the tissues.
Metabolism
Elimination of drugs occurs by metabolism and excretion. Metabolism of antimicrobials converts them to more water-soluble metabolites that are readily excreted into the urine or bile. Drug metabolism usually involves multiple pathways and a particular drug may simultaneously undergo metabolism by several competing pathways: formation of specific metabolites is determined by the relative rates and affinities of each of the parallel pathways. Metabolic conversion usually decreases or diminishes the pharmacologic activity of a drug, but depending on the structure/activity relationship for the drug target, metabolites may have similar or even higher activity than their parent compound.
Excretion
Excretion of antimicrobials or their metabolites can take place through numerous pathways (kidney, liver, lung, skin, etc.), but the most significant organ for excretion is the kidney.
Pharmacodynamics
Note that you will not be tested on this section in the end-of-course quiz.
Pharmacodynamics parameters are a measure of the ability of an antimicrobial to kill or inhibit the growth of the infecting organism. Pharmacodynamics parameters include the following:
- The time for which the serum concentration of a drug remains above the
minimum inhibitory concentration (MIC) for a dosing period T (T > MIC). This is the most important parameter for time-dependent antimicrobials. - The ratio of the maximum (peak) antimicrobial concentration, Cmax, to MIC (Cmax/MIC). This is the most relevant measure for antimicrobials whose efficacy depends on reaching a specific concentration.
- The ratio of the area under the curve (AUC) during a 24-hour period to MIC (AUC24/MIC). This can be a useful parameter for both time and concentration dependent antimicrobials.
- The post-antibiotic effect (PAE); that is, where antibiotics continue to be effective after treatment.
Figure 6 illustrates the pharmacodynamics parameters with examples of relevant antimicrobials.
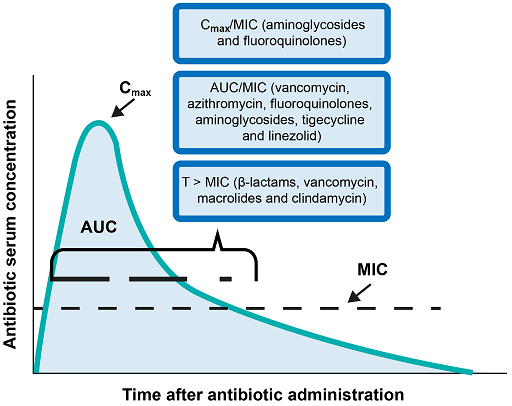
To determine the appropriate dosing levels and frequency for any antimicrobial, it is necessary to understand which parameter correlates with the efficacy of the antimicrobial. For example, the efficacy of aminoglycosides (antimicrobials that inhibit protein synthesis) correlates with AUC/MIC and also Cmax/MIC. This implies concentration-dependent killing, which means that larger doses will have an optimal effect.
β-lactams, on the other hand, require dosing to maintain the concentration above the MIC for longer periods for maximum efficacy. However, it is not that simple, as we need to understand the relationship between the therapeutic and toxic effect of drugs.
Figure 7 shows that the dose of a drug can be increased progressively until a desired response is achieved; however, if it is further increased, no additional desired effects are achieved, and unwanted effects can be seen. The orange line shows an optimal dose, above which toxicity occurs.
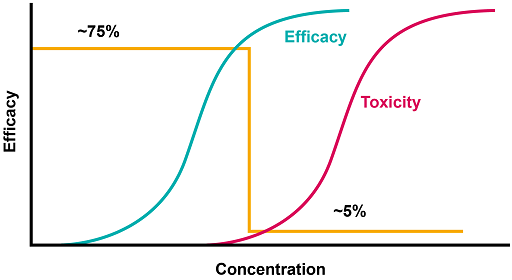
Depending on the drug it will be necessary to consider other factors such as other medications, renal function or weight.
Pharmacodynamically, the rate and extent of the bactericidal activity of an antimicrobial agent depend on:
- drug concentration at the site of infection
- bacterial load
- phase of bacterial growth
- the MIC for the pathogen.
It follows that a change in any of these factors will alter the activity of the antimicrobial agent against a particular pathogen, and may affect the outcome of therapy.
Frequency of dosing
Note that you will not be tested on this section in the end-of-course quiz.
Watch Video 2, which will remind you of the key concepts of concentration-dependent killing and time-dependent killing, and consider how this information can be used to optimise the level and frequency of dosing.
Transcript: Video 2 Pharmacology of antimicrobials for clinicians (WHO, n.d. 3).
‘Pharmacology of antimicrobials for clinicians, part 2.’
In the previous segment, we reviewed the basic concepts of pharmacokinetics and pharmacodynamics of antimicrobials, and the bioavailability of these drugs. Now, let’s turn our attention to another key pharmacology concept.
Recall that pharmacodynamics refers to what the drug does to both the human and the microorganism. A critical aspect of antibiotic efficacy is the rate of bacterial kill, and the effect of increasing drug concentrations and/or prolonged exposure on that rate. This determines whether bacterial killing by an antibiotic is concentration-dependent or time-dependent. Some antimicrobials also exhibit post-antibiotic effects: the persistent inhibition of bacterial replication after removal of the antibiotic from the system.
Knowing these properties, in turn, enables clinicians to understand and use a dosing regimen that maximises an antimicrobial’s effect. Aminoglycosides and fluoroquinolones, for example, demonstrate concentration-dependent killing over a great range of concentrations, and have post-antibiotic effects. With such antibiotics, the appropriate strategy is the administration of large, infrequent doses. Thus, the higher dose maximises killing, while at the same time, the persistent post-antibiotic effects help to maintain the antibacterial activity between doses.
The practise of once-daily dosing of aminoglycosides and some fluoroquinolones is built around this principle. Beta-lactam antibiotics, in contrast, exhibit time-dependent activity, with limited post-antibiotic effects once exposure to the antibiotic has ended. In this circumstance, the goal is to optimise the duration of exposure of the pathogen to effective concentrations of the beta-lactam antibiotic.
One microbiology concept is important to introduce before moving on. The minimum inhibitory concentration, or MIC, is the lowest concentration of an antimicrobial that inhibits visible growth in vitro, or in other words, of an organism on a plate of culture media. This is determined by testing serial concentrations of an antimicrobial in assessing for the inhibition of bacterial growth. Reference microbiology organisations, like CLSI or EUCAST, provide interpretive guidelines using these MICs to predict clinical outcomes. These organisations provide MIC breakpoints to classify organisms isolated in culture as susceptible or resistant to a particular antimicrobial. CLSI also includes breakpoints that are interpreted as intermediate susceptibility; however, EUCAST does not. For those who would like to learn more, we direct them to this video that provides guidance on reading breakpoint tables.
Let’s return to beta-lactams. Given the pharmacodynamic properties of this class of antibiotics, the goal is to optimise the pathogen’s duration of exposure to beta-lactam concentrations in excess of the MIC of that pathogen. While there is variation between different beta-lactam antibiotics and different target bacteria, a drug concentration of greater than four times the MIC for 40–60% of the dosing interval is generally sufficient to reach this goal. This prolonged exposure can be achieved by a strategy of frequent, extended,or continuous infusions. This critical concept allows us to effectively use antimicrobials, despite some marginal degrees of antimicrobial resistance; for example, organisms with an MIC just above the resistance threshold.
Let’s apply this concept to another urine culture, which also grew E. coli. But in this case, the organism was resistant to ampicillin, fluoroquinolones, trimethoprim-sulfamethoxazole and ceftriaxone. The E. coli isolates MIC to one commonly used beta-lactam, piperacillin tazobactam, is 8. According to EUCAST, this MIC is considered the upper limit of susceptibility.
However, you know that piperacillin tazobactam is a time-dependent antimicrobial. You typically dose piperacillin tazobactam every eight hours, with doses administered over 30 minutes for non-pseudomonal infections. As depicted in this figure, intermittent dosing provides an adequate time above MIC, represented by the yellow arrow, to be effective against highly susceptible organisms with quite low MICs.
However, for susceptible organisms, with higher MICs, this dosing strategy less reliably provides sufficient time for which the serum concentration of the antibiotic is above the MIC, compromising piperacillin tazobactam’s efficacy. So instead, you consider administering each drug over four hours, instead of over 30 minutes, in order to increase the time above MIC, and thus, optimising the likelihood of achieving the pharmacodynamics target and enhancing piperacillin tazobactam’s efficacy.
-
Why can it be advantageous to administer piperacillin/tazobactam over four hours instead of over thirty minutes?
-
Because this increases the time each drug is above MIC in the body, and thus optimises the likelihood of achieving the pharmacodynamic target and successful treatment.
Oral or intravenous antimicrobial therapy
A clinically unstable patient may often initially require intravenous antimicrobial therapy. However, switching to oral therapy once they are improving has big advantages, and one relatively easily achievable AMS goal is ensuring this switch is made in a timely way when this is appropriate.
Switching could be built into the prospective audit and feedback approach, or the pharmacy might take responsibility for reminding clinicians of the need for reassessment if there is no electronic prescribing system to do this.
Now watch Video 3, which has been taken from a larger WHO course on AMS, and answer the following questions.
Transcript: Video 3 Pharmacology of antimicrobials for clinicians (continued) (WHO, n.d. 3).
Bioavailability, or the amount of active drug that reaches the bloodstream after administration, is a key pharmacokinetic property of each antimicrobial. Highly bioavailable oral antibiotics are those that achieve serum concentrations comparable to antibiotics administered intravenously.
Although many hospitalised patients initially receive empiric intravenous antimicrobials due to clinical instability, the course of therapy for many common infections may subsequently be completed with orally administered drugs. Switching to oral antibiotics when appropriate can decrease costs, facilitate discharges and save patients from complications associated with indwelling intravenous catheters, including infection and clots.
In order to determine when oral antimicrobial therapy is indicated, clinicians can ask themselves the following questions:
Is the patient haemodynamically stable? If the answer is no, then intravenous antimicrobial therapy may be warranted. In hospitalised patients that had initially received intravenous antimicrobials, the clinician should assess the patient’s clinical trajectory. If they’re improving clinically, switching from intravenous to oral antimicrobials may be appropriate.
Is the patient eating or tolerating enteral feeding? Patients with persistent nausea and vomiting may not be candidates for orally administered antimicrobials.
Is the patient able to adequately absorb orally administered medications? Patients with an active gastrointestinal bleed, or those with ileus, bowel absorption syndromes or proximal resection of their small intestines may not be candidates for orally administered antimicrobials.
Is there an orally bioavailable antibiotic that could be used to treat this infection? Bioavailable oral antibiotics that are commonly prescribed in practise include fluoroquinolones, doxycycline, azithromycin, trimethoprim-sulfamethoxazole, metronidazole, linezolid and fluconazole. Some beta-lactams have good or at least adequate oral bioavailability when dosed appropriately. Some life-threatening infections, such as meningitis or endocarditis, should not be treated with oral antimicrobials.
However, many other commonly encountered infections – including community-acquired pneumonia, skin and soft tissue infections, and urinary tract infections – are good candidates for either initial empiric or conversion to oral antimicrobial therapy to complete a course. Microbiologist, infectious disease specialist and clinical pharmacist (if available) can often assist when making decisions regarding the use or transition to oral antimicrobials.
-
Name as many benefits as you can of switching from IV to oral antimicrobial therapy.
-
You might have thought of:
- decreasing costs
- facilitating discharge
- avoiding complications associated with indwelling intravenous catheters, including infection and clots.
-
What factors determine whether a patient is a candidate for a switch from IV to oral?
-
You might have suggested that the patient can be switched to oral antimicrobial therapy if:
- they are eating or can tolerate food
- they do not have any condition that affects their ability to absorb antimicrobials administered orally
- their condition will respond to treatment with an antimicrobial that is orally bioavailable.
Patients with endocarditis or meningitis should not be switched to oral antimicrobials.
Choice of antibiotic formulations
Like all other medicines, antibiotics are generally available in a wide variety of formulations, and often manufactured by different countries. It is important to choose the right one for the right patient.
In many countries, information about registered medicines is held in online databases. For example, the Electronic Medicines Compendium (EMC) contains detailed information about all medicinal products that are registered for use in the UK, including pharmacological and pharmacokinetic properties, contraindications (including side-effects), and dosing. You can also see the summary information that is available on the patient information leaflets.
If you would like to explore the EMC further, you might like to look briefly at the entry for nitrofurantoin, an antibiotic that is now often prescribed for urinary tract infections (UTIs), as an example. Increasing use of this antibiotic is driven by resistance to others that have been commonly used for these infections, such as fluoroquinolones. You will see a wide range of different formulations and dosing regimens for oral delivery.
De-escalation
Summary of patient-level interventions
Figure 8 sums up the application of antimicrobial stewardship to an individual patient.
Choose the correct statement(s) from the following list. Correct use of antimicrobials is based on following:
a.
the antimicrobial’s mechanism of activity
b.
whether the effect of the drug on the bacteria is bacteriostatic or bactericidal
c.
the mechanism of resistance to the group of antibiotics
d.
pharmacokinetic parameters
e.
pharmacodynamic parameters.
The correct answers are a, b, c, d and e.
5.3 Summary of interventions
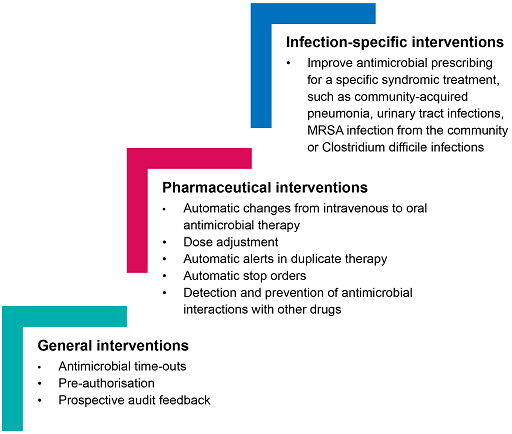
Figure 9 summarises some of the interventions mentioned in the previous sections. It also notes that interventions can be at the level of the infection itself, which is beyond the scope of this course.
Activity 6: Reflecting on interventions
Were you already familiar with some of these interventions?
Make a note of any strategies that were new to you, and for those you noted, think about how you might change your practice, at facility or individual level, to apply what you have learned. You may want to return to any sections that particularly interested you to add detail to your ideas.
6 Diagnostic stewardship and the role of the laboratory
The laboratory plays an essential role in identifying pathogens and detecting AMR. The importance of good communication between the laboratory and clinicians in clinical decision-making is covered in the Diagnostic stewardship in clinical practice course.
7 Monitoring
AMS programmes should select the most relevant and feasible metrics to monitor for the specific objectives. For example, one objective of AMS is to ensure access to effective and affordable antimicrobials; an indicator for this objective is the percentage availability of these antimicrobials. This can be reported periodically at regular AMS committee meetings. Another indicator for institutional AMS is the percentage compliance to antimicrobial prescription guidelines.
There is no value in monitoring or auditing without timely feedback to managers and health workers at unit or ward level. Regular feedback promotes best practices and, over time, results in behaviour or system change towards improved quality of care and patient safety, with a goal to reduce AMR.
Changes can be made in response to feedback aiming for a better understanding of patients’ clinical needs and treatment options, and the effects of those changes can themselves be monitored and fed back to assess whether interventions are effective, and where further improvements are indicated. In this way, individual prescribers also learn by using the feedback and are engaged in the overall process.
Impact indicators help in the setting up of governance structures, policies and strategic elements such as the national surveillance systems and processes for the AMS strategy framework. Impact indicators for AMR strategy and implementation include percentage reductions in:
- key resistant organisms
- national consumption of antimicrobials linked to key resistant organisms
- maternal mortality from infectious diseases
- neonatal mortality from infectious diseases.
Figure 10 gives an overview of the steps involved in implementing AMS in LMICs.
7.1 Quantity measures: antimicrobial consumption
- the number of units
- the number of prescriptions
- by the physical mass or volume of drugs.
Consumption may be an estimate based on import information, or sales information from pharmaceutical companies to hospitals and pharmacies. It is often provided as a rate dividing consumption by the opportunities for AMU: for example, per 100 bed days for hospital inpatients.
A common way to express consumption totals is the ‘
- Number of DDDs = Total grams used ÷ DDD value in grams
Now watch Video 4, where a pharmacist, Mrs Opanuga, discusses what stewardship is and how it started in her location.
Transcript: Video 4 A pharmacist’s perspective on AMS (FutureLearn, n.d. 2).
DDD is ‘defined daily doses’, and the main purpose of this DDD system is a tool for presenting drug utilisation statistics with the aim of improving drug use. It’s a WHO tool that was developed, and it is defined as the assumed average maintenance dose per day for a drug for its main indication in adults, assuming that compliance is there. So it’s a technical drug metric system that measures drugs consumption. And we can use it to standardise comparison with drug use between different drugs, between different [inaudible], between countries.
Like I said, it does not really – it’s a unit of measurement, but it does not reflect the prescribed daily dose. It’s not the same thing. They are different. They are different. So we can’t – you know, you can use it to calculate the drug consumption, cost of drugs, and also to study adverse – the frequency of adverse drug reaction.
In addition to what I said, DDD can be used to collect utilisation data in various settings. You can use it to collect sales data from the wholesale, dispensing data from the pharmacy with electronic or manual. You can use it to collect patient and contact-based data, patient survey data, health facility data, and so on and so forth we should note that DDDs are used to measure antibiotic use over time and are probably used by AMS team to monitor the trends within the ward, within the hospital, or a primary care setting. This allows the team to identify areas of other investigations and use audits and quality improvement methods to address such.
-
Is the DDD the same as a prescribed dose?
-
No. The DDD is a standardised measure that allows data to be easily interpreted across hospitals and countries, but does not reflect a dose that is necessarily prescribed to an individual patient.
Watch Video 5, where Aalaa Afdal explains how to use quantity measures, and then answer the questions below.
Transcript: Video 5 Quantity measures (FutureLearn, n.d. 3).
Quantity measures is the measures of the aggregate antimicrobial use. And it’s the most common metrics for the ASP. And they are often expressed as a rate x divided by y, as we mentioned before. By using a standard approach to recalculations, antimicrobial stewards are in a better position to make meaningful comparisons across locations where antibiotics are used: hospital, wards, patients.
There are many numerators that we can use, like defined daily dose, days of therapy, length of therapy, prescribed daily dose, and others. What's the difference between days of therapy and length of therapy? Days of therapy are the number of days that a patient is on antibiotic, regardless of the dose. And it’s applicable for paediatrics – that’s a good point. For example, if we have a patient who is on two antimicrobials: he started his first antimicrobials on Day 1 and Day 2, then stopped. While from Day 2 to Day 5, he used another antimicrobial, which is Antimicrobial 2. So the days of therapy for the first antimicrobial for this patient are two days of therapy. While for the second are only four. So the total days of therapy of antimicrobials for this patient are six days of therapy. But what about length of stay? Length of stay are the duration when he started his first antimicrobial regimen till the end of the last antimicrobial drug used. Here, the length of therapy are five days.
Days of therapy can be used to target the consumption of certain antimicrobial, meropenem, or certain class, like carbapenems, or even certain formulation. And it depends on your goal. Defined daily dose can be used too. But days of therapy can be measures also to assess some ASP strategy’s implementation, such as unnecessary double coverage, such as redundant anaerobic coverage of ertapenem and metronidazole, or compliance to antimicrobial duration therapy. And this can be clear from days of therapy. So days of therapy are used to assess some prescribing habits for wards or institutions as well. It’s advisable to review resources attached for further details on antimicrobial use metrics.
What’s a prescribed daily dose? It’s average prescribed dose in the main indication defined locally at ward, or hospital, or even a group of hospital level, but it does not allow inter-hospital comparisons. What about the defined daily dose? The defined daily dose is assumed average maintenance dose per day for a drug used for its main indication in adults. Which means that, for example, the paracetamol defined daily dose is 3 g. That means that the average maintenance dose (not the loading one) used for an adult patient who weighs 70 kg of paracetamol used as an antipyretic is 3 g daily.
A defined daily dose will only be assigned for drugs that already have an ATC code. ATC code consists of many items – a letter, the number, then another letter, then another letter, then another number – that we’ll see in a few seconds. But it should be emphasised that the defined daily dose is a unit of measurement and does not necessarily reflect the recommended or prescribed daily dose. Doses for individual patients and patient groups will often differ from the defined daily dose, and will necessarily have to be based on individual characteristics – age, weight and so on – and also pharmacokinetic considerations.
Defined daily dose are not established for topical products, sera, vaccines, antineoplastic, allergens extract, general and local anaesthesia, and also contrast media. So now, I will show you briefly how to navigate and get a specific DDD for a given drug formulation.
When you first opened the website for the WHO ATC/DDD, you will have a page like this. When you click ‘DDD’ on your left, you will have more information about the defined daily dose. When you click the ‘ATC/DDD index’, you will have this page where you can search for the DDD by the ATC code or the drug name. Obviously, searching by scientific name is much easier.
So we type, for example, ciprofloxacin. We will have all ciprofloxacin available. The code J is for systematic use of drugs; the number 1 is for antimicrobial class; the letter M is for quinolones; the letter A is for fluoroquinolones; finally, the number 2 is for the ciprofloxacin itself. That’s used as an anti-infective systemically without any combination.
We will choose the first one. We will have the following. As we see, the defined daily dose for oral cipro is 1 g, while that for parenteral use is 0.5. So we have to be very careful. Finally, don’t forget that the DDD is updated annually. And the last update is written below.
How to calculate defined daily dose? First step is to determine the number of DDDs of each antibiotic, which will be by the total grams dispensed, number of packages multiplied by a number of units per pack multiplied by concentration divided by the DDD defined by the WHO collaborating. This is the numerator data. Then you have to divide by a denominator data, as we mentioned before.
Some examples: you can see here ciprofloxacin, 500 mg tab, 20 per tab, 100 packages. So the number of grams are 100 packages multiplied by 20 tablets per pack multiplied by 0.5 g per tab, which is equal to 1000 g. Then the number of DDDs equals 1000 divided by 1, which is the DDD defined by WHO collaborating centre. So the total DDDs for ciprofloxacin to be taken orally are 1000 DDDs for this example.
What are advantages? Advantages are that they are independent from price and package size, easy, allow for comparisons, and it can define that one day treatment receives approximately equal weight, whatever the drug is. For example, 0.24 g gentamicin is approximately equal to 4 g cefotaxime, approximately equal to 14 g piperacillin.
But limitations: it is not suitable for paediatrics and patients with organ dysfunctions; it’s updated annually – we have to keep an eye on it; and the total DDDs is strongly influenced by formula mix.
-
What are the uses and advantages of
days of therapy (DOT) as a measure? -
- It can be used to identify unnecessary double antimicrobial coverage.
- It can check that prescribing is compliant with guidelines on duration of therapy.
- It is applicable to paediatric patients as well as adults.
-
How would you approach calculating the DDD?
-
You can calculate the total grams dispensed by considering the dispensed number of packages, multiplied by the number of units per pack, multiplied by concentration. Then divide this quantity in grams by the DDD defined by the WHO.
7.2 Quality measures: AMU and point prevalence surveys
A
PPSs may also be used to collect information specific to hospital-acquired infections. For AMU, PPS collects information on prescribing practices for antimicrobials, and other information relevant to treating and managing infectious diseases in hospitalised patients. This data complements the surveillance data on antimicrobial consumption. As shown in Figure 10, the results of PPS can be used to:
- evaluate quality indicators
- follow up antimicrobial stewardship and infection control programmes
- support decision-making.
Point prevalence is defined as the proportion of people with a particular characteristic at a certain point in time. It is determined by taking the total number of people with the characteristic divided by the total number of people in the population of interest. An AMU PPS survey measures the number of people taking antimicrobials at a given point in time.
Although we are talking about PPS at the end of the course, it is a tool that could be used effectively when initiating an AMS strategy; and if repeated at regular intervals, it can drive the evolution of AMS and IPC.
Specific goals of an AMU PPS are to:
- estimate the prevalence of AMU
- describe patients, invasive procedures, infections (sites, microorganisms including markers of AMR) and antimicrobials prescribed (compounds, indications) to treat the infections or provide prophylactic cover for the procedure
- describe types of patients, specialities or healthcare facilities, and how antimicrobials are used at patient and facility level
- disseminate results to those who need to know at local, regional and national level
- raise awareness of patterns of AMU among staff
- enhance surveillance structures and skills
- identify common problems and set up priorities accordingly
- evaluate the effect of strategies and guide policies for the future at the local, national and regional level (repeated PPS)
- provide a standardised tool for hospitals to identify targets for quality improvement.
A PPS is a qualitative approach: it analyses not only the quantities of antimicrobials used (as DDDs), but also which patients are receiving them, their indication, and whether the antibiotics prescribed are in accordance with local prescribing guidelines.
It should collect information on every patient in a hospital at a time point. Ideally this might be done on the same day – but this is unlikely to be practical, so different wards can be visited for collection over a specified time period, possibly a few days or weeks.
You will need to ensure that hospital management understand and support the survey, and staff will need to be made available and be trained to carry it out. Data is often collected on paper forms but will need to be entered into a database for analysis, so resources must also be allocated to this. Online tools are also available for data collection if smartphones are available.
A standardised global PPS web-based tool is provided by the Global-PPS programme, which co-ordinates regular one-day surveys in participating hospitals around the world.
Activity 7: Looking at Global-PPS
Explore the Global-PPS website, and look at an example of a PPS form. You may think that your hospital is not ready to join the global survey yet, but there are still some useful resources available for your own PPS.
Is the PPS survey intended to change the clinical management of individual patients?
Answer
No. The instructions specifically say that discussion of the appropriateness of antimicrobial treatment of individual patients is not permitted. The aim is only to take a snapshot of the quality of AMU in the hospital.
A WHO document details its methodology, and how facilities can calculate the indicators themselves, rather than entering the data into the global database (WHO, 2018).
An example of a national PPS
Nepal has carried out a national AMU PPS series to analyse the lack of rationale antimicrobial use in Nepalese hospitals. The first of its kind in Nepal, the AMU survey covered six hospitals and generated data on AMU in hospital settings. Analysis of the data showed a high prevalence of AMU within the AWaRe classification framework. The findings drew attention to the importance of initiating AMS programmes and AMS activities now take place in the surveyed hospitals.
(You can read more about the Nepal AMU PPS and its impact on health policy.)
8 Processes for improvement
Now that you are aware of both AMS interventions and methods to monitor their success, your facility needs to develop a process to identify and apply those with most potential for success in your situation, and to evaluate their efficacy.
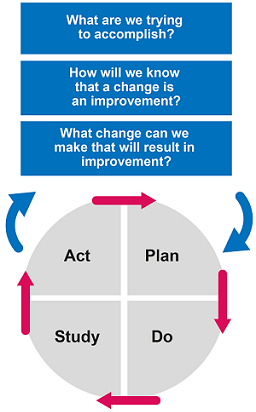
The Institute for Healthcare Improvement’s Plan–Do–Study–Act (PDSA) cycle can be useful for setting goals and measures of success. It is important to choose the AMS strategy best suited to the resources and situation at your healthcare facility.
Activity 8: The Plan–Do–Study–Act (PDSA) cycle
Look at the Plan–Do–Study–Act (PDSA) cycle and add your own ideas to the model for improvement in Figure 11.
Discussion
At this stage your thoughts might be quite general, because it is necessary to form a team that brings together expertise from your hospital to identify a plan that will suit your situation. You might have thought of the following:
- Setting aims: To reduce the use of Reserve and Watch group antimicrobials in your hospital; to improve understanding of the situation in your facility by establishing surveillance for AMR and AMU.
- Establishing measures: You will need to assess the current use of antimicrobials in order to formulate a specific time-limited goal. PPS is one method to do this.
- Selecting changes: Consider the appropriate strategy. You might wish to talk to your colleagues in other hospitals, who may have some experience of these processes. You may have suggested introducing prospective audit and feedback, or working on involving different hospital staff and providing education on AMR to begin to build a positive culture to address it. You may also have considered revisiting the principles of diagnostic stewardship and investing in your laboratory facilities to support AMS.
9 Education and training
Antimicrobial Stewardship: A Competency-based Approach is a free course created by the WHO for clinicians that provides detailed information on appropriate treatment for specific conditions in separate short courses. It aims to address the following core competencies:
- C1: Understands the patient and the patient’s clinical needs
- C2: Understands treatment options and how they support the patient’s clinical needs
- C3: Works in partnership with the patient and other healthcare professionals to develop and implement a treatment plan
- C4: Communicates the treatment plan and its rationale clearly to the patient and other health professionals
- C5: Monitors and reviews the patient’s response to treatment
The courses in the WHO course are specific to different types of infection. If you are a clinician, you may find some of these useful to develop your knowledge of AMS further.
Activity 9: An example of an AMS
Make notes as you watch Videos 6 and 7, and then answer the questions below.
Watch the following interview video on an example from Nigeria.
Transcript: Video 6 An example of stewardship from Nigeria (FutureLearn, n.d. 4).
I’m Dr Iretiola Fajolu, a consultant paediatrician with the Lagos University Teaching Hospital, a member of the hospital antimicrobial stewardship committee, and chairman of my departmental antimicrobial stewardship committee. I’ll be talking about when and where to start using that experience in the department.
Where to start: involving the key stakeholders is important to ensure a successful programme. It is also important to identify the members of your AMS team and keep them informed. It is necessary to have a baseline data to use as a starting point to identify the need for stewardship, and also to measure the success of the programme.
The hospital antimicrobial stewardship committee made a presentation of the results of the global point prevalence survey of antimicrobial consumption and resistance to all members of the departments. This helped create awareness of the problem of high rates of antimicrobial use in the hospital. A case presentation was also done, which highlighted the gaps in the routine investigations and antibiotic management of infections in our department. The commitment for an intervention to improve antibiotic prescribing was obtained from our members, and the Departmental Antimicrobial Stewardship Committee was settled.
The members of the department also decided on the stewardship strategy to use, and prospective audits, intervention and feedback was agreed upon. The duties of the Departmental AMS Committee were to draw up an antibiotic guideline, and also to monitor compliance to the guideline and the hospital antibiotic policy.
Our first challenge was developing the antibiotic guideline. There was no antibiogram to base our choice of antibiotics on, so the first draft was based on existing guidelines from other countries, and also on a knowledge of common causative agents and the likely sensitivity. The initial draft was sent to individual members of the department for input, but the response was poor. It was then resent, this time asking for input from units instead of from individuals. This resulted in a better response.
The final draft was then sent out and we asked that it be used in the department. This process took some time, and it was almost discouraging. This also coincided with the dissemination of the hospital antibiotic policy. Our next duty was to monitor compliance to the antibiotic guideline and also to the hospital antibiotic policy. The areas of focus were targeted prescription, closed prescription, de-escalation, collection of culture samples before commencing antibiotic therapy and also the use of biomarkers for diagnosing infections.
The next challenge however, was manpower to carry out prospective audits, intervention and feedback. We decided to do a study to assess the feasibility of involving medical students for data collection on antibiotic use with the help of a checklist to reduce the work burden for healthcare professionals. This was a retrospective review of case notes.
It afforded the medical students early training and practice in rational antibiotic use. This was found to be feasible, and was incorporated as part of the training of medical students in the department. It is now being done as a prospective audit. The aspect of feedback is, however, yet to be assessed, as the programme is still in the early phase or in the departments. Thank you.
Transcript: Video 7 A pharmacy perspective (FutureLearn, n.d. 4).
Oh, there are the challenges. We are working in low-resource settings. And we have the three Ms – are never sufficient – manpower, money, material. They are not sufficient. So we have to manage where we are. But even that as well: shortage of manpower is key. If there is nobody to go on a ward round, a robot cannot do institution, make intervention. So we need manpower. We are very short. And it’s actually affecting hours. So we need manpower.
Multidisciplinary ward rounds should be improved on. It’s not adequate yet. We see mission some challenges when we go to the world, inadequate review of, or untimely review of, hospital formulary. When an hospital has been there for more than ten years, new drugs are not brought on board, you know? So it’s affecting to affect the AMS.
Failure or litanies of review medication errors. Where we see some intervention, we see some errors and we want to – if it’s not done on time, or we cannot reach the prescriber, the patient will not start their medication on time. And that will affect rational use of the antibiotics.
Open prescription, opened-up prescription for antimicrobial agents and other drugs; lack of communication between prescribers and pharmacies – where we are not really communicating, they use or lose sheets or wrong forms to document administration of IV drugs, which are not even filed in the case notes. And so we cannot really follow up on use. Poor documentation of drugs is that our documentation of drugs not administered, and no dissemination of relevant information to our stakeholders.
- How did the AMS team assess the situation at the beginning of Video 6?
- What challenges did they have in developing an antimicrobial guideline and how did they address them?
- In Video 7, what challenges did Mrs Opanuga identify?
Discussion
- The AMS team assessed the situation with a PPS, which they communicated to all relevant staff.
- There was no antibiogram, so the initial guideline was based on guidelines from other countries, adapted using local knowledge. Additionally, when individuals were asked to give feedback, the response was poor. The request was sent to units, and better feedback was obtained.
- You may have recalled that she mentioned:
- a lack of manpower
- new drugs not being added to the formulary
- late reviews of medication
- lost forms or incomplete documentation
- patients starting their medication late due to errors or poor communication between clinicians and the pharmacy.
Activity 10: Reflecting on using an AMS
Now that you have completed the course, can you identify any simple ways that AMS could be improved at your own healthcare facility? Can you think of any changes you might make in your own practice?
Discussion
At the hospital level, you may have thought of better communication of relevant data (such as up-to-date hospital antibiograms) and better training (such as continuous in-service training to keep all professionals up to date on appropriate choice, doses and timing of antimicrobials).
In your own practice you might have thought of timely switching from IV to oral administration. You may also have decided to study WHO resources relevant to your prescribing practice, such as selected topics from Antimicrobial Stewardship: A Competency-based Approach (WHO, n.d. 3).
10 End-of-course quiz
Well done – you have reached the end of this course and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
11 Summary
In this course you have learned about the importance of AMS structures, and the strategies needed to implement AMS at a national and hospital level. AMS programmes are multidisciplinary initiatives that should be integrated into all areas of healthcare; but, because AMR is a global problem, stewardship should be extended to diverse areas including veterinary and agricultural AMU, in line with the One Health approach.
AMS evolves over time in a cycle of measurement, feedback and change, which allows it to adapt quickly in the ongoing fight against bacterial pathogens and the development of AMR. By investing in AMS – whether starting from nothing or building on an existing programme – a hospital may improve patient care and outcomes, save money and reduce the development of AMR.
While this course has explained how AMS can involve resources such as integrated IT systems and modern laboratory testing techniques, these resources are not available everywhere; in low-resource settings, small steps towards AMS can still result in significant benefits. Essentially, it must be underpinned by effective collaboration and communication between clinical, laboratory and pharmacy staff, and managed as an essential activity, with education and training.
You should now be able to:
- understand the principles of an AMS programme
- understand how to approach setting up an AMS committee
- indicate the place of the laboratory in the AMS process
- develop local prescribing guidelines
- describe AMS strategies such as prospective audit and feedback
- understand quantitative and qualitative ways to assess antimicrobial consumption and use
- appreciate the importance of understanding the pharmacokinetics and pharmacodynamics of antimicrobials in determining the optimum treatment strategy
- appreciate that AMS can be introduced gradually, and that initial emphasis on easier-to-achieve targets can lead to significant improvements
- refer to treatment guidelines and resources (such as WHO courses)
- describe the costs and benefits (cost-effectiveness) of implementing AMS.
Now that you have completed this course, consider the following questions:
- What is the single most important lesson that you have taken away from this course?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 11: Reflecting on your progress
Do you remember at the beginning of this course you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this course, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this course, go to your reflective blog and note down your thoughts.
12 Your experience of this course
You’ve now reached the end of this course. If you’ve enrolled on a pathway, please go back to the pathway page and tick the box to confirm that you’ve completed this course. On the pathway page you’ll see both your progress so far as well as the other courses you need to complete in order to achieve your Certificate of Completion for that pathway.
Now that you have completed this course, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this course and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Acknowledgements
This free course was collaboratively written by Olga Perovic and Dawn Harmon, and was reviewed by Priya Khanna, Claire Gordon and Hilary MacQueen. The course was reviewed and updated by Priya Khanna, Hilary MacQueen, Clare Sansom and Rachel McMullan in 2025.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Images
Course image: gorodenkoff/iStock/Getty Images Plus.
Figures 1, 3, 5 and 8: courtesy of Professor Olga Perovic.
Figure 2: World Health Organization, https://adoptaware.org/.
Figure 4: McKinnon and Davis, 2004.
Figure 6: Al-Dorzi et al., 2013.
Figure 7: Padmanabhan, 2014.
Figure 9: Afdal, A. ‘Quality measures’, taken from https://www.futurelearn.com/ info/ courses/ antimicrobial-stewardship-for-africa/ 0/ steps/ 50501.
Figure 11: model for improvement taken from ‘Science of improvement: how to improve’, http://www.ihi.org/ resources/ Pages/ HowtoImprove/ ScienceofImprovementHowtoImprove.aspx.
Text
Activity 7: Global-PPS, https://www.global-pps.com/.
Tables
Table 1: Truong et al., 2021.
Videos
Videos 1 and 4–7: British Society for Antimicrobial Chemotherapy (BSAC), https://www.bsac.org.uk/.
Videos 2 and 3: WHO, n.d. 3.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.