Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 22 February 2026, 1:58 AM
Module 2: Longevity and viability
Introduction
Welcome to the second module of this online course. In module 1, you learned about the development and overall goals of seed genebanks, and considered the FAO’s Genebank Standards and Practical Guide: their uses and their limitations.
In this module, you will first consider seed longevity. It is generally recognized that the longevity of seeds increases as moisture content and temperature decline. However, scientists are still trying to figure out the optimal combination of these conditions. The CGIAR genebanks have much to contribute here, since CGIAR genebanks have built up a bank of evidence in their collections.
Longevity is closely related to the concept of viability, the second focus of this module. You will explore influences on longevity and viability, both in orthodox seeds and in species whose seeds are more challenging to store. By the end of this module, you will understand what factors can influence viability and how you can use them to improve outcomes. This will put you in a good position to apply what you have learned in your own work setting. We will give you the opportunity to read about the science of longevity and viability in more depth in our ‘Useful publications’ section.
By the end of this module, you should be able to:
- Describe how seed longevity is measured.
- Sketch graphs to show the predictable patterns in decline of seed lot viability over time.
- Define the terms p50 and p85, and how they are used.
- Give examples of the impact of changing moisture content and temperature on seed longevity.
- Explain how the daily activities of genebanks can affect viability, and how genebanks can monitor viability effectively.
- Explain how genebanks can improve their practice by learning from seeds that do not germinate.
- Evaluate the procedures in your own workflow.
Overview of genebank processes
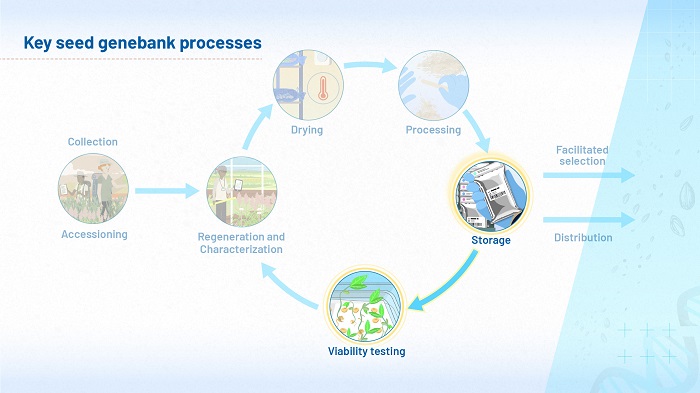
Figure 1 (above) highlights the two processes, storage and viability testing, that we will be considering most in module 2. They are set within our process model of the ex situ conservation of seeds in genebanks.
The cycle, which you first saw in module 1, is sometimes described as ‘steady state operations’. Enough seed is regenerated, dried and characterized to ensure that the amount of plant genetic resources for food and agriculture (PGRFA) in storage will serve the needs of users. In module 1, you learned that seeds age during the time they spend in storage, so in order to maintain steady state operations, it will eventually be necessary to regenerate. Viability testing is carried out regularly, to ensure that sufficient PGRFA is regenerated, and the cycle starts again.
In this module, you will discover some of the factors that can influence seed aging during storage and some of the mechanisms involved. How well seeds are dried and sorted before they go into storage can also make a difference to their viability when they come out of storage. Viability testing is therefore crucial to a genebank’s success. While some of the influences on seed longevity may be beyond your control, the data yielded by regular viability testing will ensure that your stock is regenerated in a timely way.
Some definitions
In module 1, you learned some basic definitions of longevity and viability. Now let’s dive deeper into how scientists find out about these, and what they have learned. First, here are a few definitions of ways you can find out about a seed’s longevity and viability:
The time taken for viability to fall to 50%, p50, is often used as a measure of seed lot longevity.
The length of time spent in a given storage environment is the storage period, p.
A germination test involves growing-out samples of seeds on wet paper or another medium such as agar, moist soil or sand.
The topographical tetrazolium staining test is a destructive test, which relies on the fact that viable tissue will react with the tetrazolium solution and therefore stain red, whereas non-viable tissue does not react.
In seed storage experiments to understand the relative longevity of different seed lots, seeds are subjected to high temperatures and humidity prior to testing germination. This can be used to assess seed lot vigor.
Why does seed longevity matter?
Genebanks are at the forefront of conserving the world’s genetic diversity. They provide agricultural researchers with the PGRFA they need to address problems of food security and climate change. For these reasons alone, genebanks expend an enormous amount of effort to ensure the survival of seeds in their care.
Watch this video, in which scientists and genebank managers discuss the measures they have adopted to secure the longevity of seeds in their genebank. The video was filmed in two particular genebanks, IRRI and IITA, but the underlying biological mechanisms that are referred to are common to many crops and relevant to many other settings, including yours.
The video considers how to minimize aging reactions while seeds are in storage. As you watch, think about what underlying chemical reactions the scientists are attempting to control in their bid to maximize seed longevity.

Transcript: Video 1: Securing seed longevity
Please write your comments on how seeds are prepared for storage, what reactions can cause seed to age during storage, and what genebanks can do to slow these aging reactions down. You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
Fiona Hay and Olaniyi Oyotomi discuss mechanisms by which seeds may age, lose vigor, and die in storage. The first thing to consider is the maturity of seeds: seeds that are immature when they go into storage will have low viability when they come out. Sorting seeds well before they go into storage can avoid this. Two factors associated with aging are moisture and oxygen from the surrounding air. Drying seeds before they go into storage helps the genebank control moisture. Packing them in sealed, vacuum-packed aluminum envelopes prevents both moisture and oxygen from reaching the seeds.
How viability changes over time

As you discovered in module 1, the rate at which seeds lose viability varies between species. If your genebank happens to be looking after crops like date palm, you might be able to wait hundreds of years before loss of viability becomes a problem. Beans like Phaseolus vulgaris (above) are also pretty easy to store over long periods of time.
For other crop species with medium longevity (e.g.: maize), the length of time genebanks have been in operation is getting close to the length of time those species are expected to remain viable. For crops like this, we may be approaching a new type of ‘steady-state’ operations, where more seeds need to be regenerated, as the viability of older samples in long-term storage becomes a concern. If, on the other hand, you are responsible for short-lived seeds, such as Lactuca, you already need to be alert to the risk of rapid decline in viability.
Whatever species we are talking about, scientists have used germination tests to show predictable, statistical patterns in the decline in viability. These patterns take the guesswork out of decisions about when to regenerate, and make it much easier for genebanks to manage the quality of their seeds.
Patterns in viability loss
Seeds lose their ability to germinate over a period of time. The histogram in Figure 2 shows numbers of seeds dying (the orange bars) against the length of time those seeds have spent in storage. Below the horizontal axis are descriptions of how the remaining seeds - those which have not yet died - germinate:
When first grown-out, there are no dead (i.e.: non-viable) seeds. While seeds have only been in storage for a short time, their germination, when taken out of storage, is fast and uniform. After this initial period, the number of seeds dying increases rapidly, reaches a maximum, and tails off after most of the seeds have died. Even among seeds that are still alive and able to germinate, the results become poorer as time goes by. The longer the seeds spend in storage, the greater the likelihood that germination tests will show sporadic or abnormal germination.
This same data can be converted into a graph plotting percentage of seeds germinating against time spent in storage, as we have done in Figure 3, below. In this case, you get an S-shaped seed survival curve. In the first few days, the seeds maintain most of their ability to germinate, then the loss of viability goes into a steeper decline. This decline slows back down again once the majority of seeds have already lost viability, with just the final few seeds still clinging on to viability. Eventually, none of the seeds in the seed lot will germinate.
Activity 1
Allow ten minutes for this activity.
Imagine what it would be like if instead of a smooth curve, the viability dropped in a straight line like this. To what extent would you be able to ensure that the seeds you pass on to users are viable?
Use the text box below to write down what your job would be like if seeds behaved like the graph in Figure 4.
When you are ready, press ‘reveal’ to see our comments.
Discussion
It would be very difficult! You would need to regenerate before viability drops from one hundred percent to zero. If you miss the turning point, your recipients might receive a seed lot with zero viability. They may not be able to breed from the samples you have sent out, and their experiments would be ruined. From your genebank’s point of view, there would be more risk of losing the genotype forever. The S-shaped curve, by contrast, gives warning that your stock may be losing viability, at a point when you can still do something about it.
Calculating p50
The measure of longevity is p50: the time taken for the seed lot viability to fall to 50%. Since p50 will vary with every seed lot, let’s start by looking at what happens in a fictional seed survival experiment:
Table 1 (below) shows the full set of data. Note that the third column of the table shows the number of seeds dying between sample times, rather than the cumulative number of non-viable seeds:
| Time elapsed (days) | Viability (% germination) | Number of additional non-viable seeds |
| 0 | 100 | 1 |
| 2 | 99 | 3 |
| 4 | 96 | 6 |
| 6 | 90 | 12 |
| 8 | 78 | 18 |
| 10 | 60 | 23 |
| 12 | 37 | 19 |
| 14 | 18 | 10 |
| 16 | 8 | 5 |
| 18 | 3 | 2 |
| 20 | 2 | 1 |
| 22 | 0 |
Table 1: results of viability testing
In order to calculate p50, you simply plot these data as a seed survival curve. Then read across from the point the germination on the vertical axis is 50%, to the time this has taken on the horizontal axis. It will look something like Figure 5 (below).
Whether you are dealing with species whose survival is measured in days, years or decades, this measurement, p50, is useful to compare the longevity of different seed lots. But it is also possible to work out the time taken for 75% of the seeds to lose viability (p75), the time taken for 85% of the seeds to lose viability (p85), or any other percentage. They are all worked out in a similar way.
Viability thresholds

What you have read so far should have convinced you that the time a seed lot has spent in storage is important to whether the seeds are likely to germinate. You have seen how viability tails off, and that it can do so quite quickly after an initial, relatively stable period. This loss of viability is most clearly visible after germination drops below 85%, as shown in Figure 6.
Conveniently, the Genebank Standards suggest that you should test for viability every one third of the time predicted for viability to fall to 85%, depending on the species. This gives you the best chance to regenerate efficiently, to ensure that the seeds you send out to your users will germinate, and to ensure that none of the diverse genotypes you are hoping to conserve are lost.
If the viability threshold were much higher than 85%, genebanks would need to expend more effort in constantly regenerating their stock. If the viability threshold were lower, users, who may not be sowing under the optimal conditions that genebanks can achieve, may receive seeds they cannot grow. The length of time seeds have spent in storage is therefore a good place to start when deciding when to regenerate. However, the time spent in storage is not the only factor you need to consider: there are other things that also affect seed longevity.
Moisture content
The moisture content of seeds when they first go into storage can have a significant influence on their viability when they come out of storage. Take a look at Figure 7 (below), which shows the difference in expected survival when seeds at four different moisture contents are placed into storage.
Figure 7 shows the expected survival curves – the pattern of decline in viability over time – for chickpea (Cicer arietinum) seeds when stored at 5°C.
If the chickpea seeds are dried to equilibrium at 15% relative humidity and 15°C, we expect them to reach a moisture content of 6%. As you can see, the rate of viability loss at 6% moisture content is very slow. We can predict these seeds will reach the viability threshold of 85% after 114 years. However, if the seeds haven’t been dried sufficiently, they lose viability much more quickly. The 85% viability threshold would be reached after 28 years for seeds that have been dried to 8% moisture content, 9 years for seeds dried to 10% moisture content, and just 4 years if the seeds have only been dried to 12% moisture content.
This emphasizes the importance of seed drying, which you will learn more about in module 4.
Temperature
We can also predict survival curves for seeds stored at different temperatures. Figure 8 (below) shows the effect of storing chickpea seeds at 6% moisture content in medium-term storage (5°C) and in long-term storage (-20°C). Over a period of 280 years, you can see the survival of the seeds in medium-term storage starting to tail off. For seeds in long-term storage, it looks like they’re barely losing viability at all, but they are – just very slowly!
The fact that there are there are differences in survival as a result of temperature means that it is important, when recording seed viability in storage, that you specify the temperature at which the seeds were stored, as well as their moisture content.
Dormancy
You learned in module 1 that some seeds may not germinate because they are dormant. These dormant seeds are operating on minimum metabolism, and even if environmental conditions are favorable, something else needs to happen to break their dormancy.
Dormancy can have an impact on perceived viability. If you expect viability to tail off over time in an S-shaped curve, the viability of a brand new, fresh batch of certain types of seeds can appear a little disappointing. This is because dormancy prevents some seeds from germinating, creating a false impression that they are not viable, when in fact they may just be dormant. This is common in wild species, where dormancy is a functional response to the problems of living in situ. It may prevent tender young seedlings from emerging during seasons when conditions are at their harshest. Even in an ex situ genebank setting, it is important to be alert to the possibility of dormancy.
Figure 9 shows how the changes in germination rate over time may look for a species that exhibits dormancy:
Later in this course, you will find out more about the mechanisms behind dormancy and discover ways of breaking it. But at the moment, it is worth noting that it is possible for dormancy to mask the true viability of a seed lot.
Differences between species
There are very large differences in longevity between crop species. Researchers and CGIAR genebanks have been recording them for decades. This information, shared in the literature, gives us confidence that we know how long it takes for seeds of a particular species to lose viability in storage, allowing genebanks to set realistic schedules for regeneration.
You have already seen that we are now reaching the point where, for many short-lived species, we are starting to approach the ideal ‘steady state’ for genebanks, as illustrated in module 1, and reproduced here.
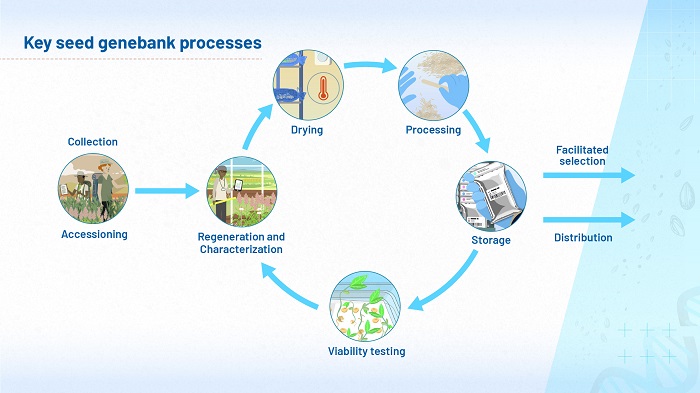
However, for the most long-lived species, even the CGIAR genebanks have not been around for long enough for us to observe directly how long it would take for viability to drop to 85%. For these species, which potentially can take thousands of years to lose viability, we have to rely on predictions. Figure 10 (below) shows the predicted times it might take for viability to fall from 98 to 85% if the seeds are stored under long-term genebank storage conditions (in moisture-proof containers at -20°C after drying to equilibrium with 15% RH and 15°C).
Like the longevity of any organism, the longevity of seeds in storage can be influenced by a number of factors. So far, we have considered time spent in storage, differences between seeds in a population, moisture content, temperature, and differences between species. This is not just of academic interest: it is important scientific knowledge that can improve your ability to do your job.
Manipulating longevity during storage
Commercial seed producers have known for some time that deliberately altering the moisture content of seeds can affect their viability. A number of methods can be used to achieve this, ranging from soaking seeds in pure water to applying chemical agents in order to adjust moisture content. For instance, seeds that are rehydrated then dried again just prior to germination can show more rapid, uniform germination – this process is called ‘priming’.
Until recently, this knowledge has had limited uptake in genebanks. There were fears that the lifespan of primed seeds in long-term storage may be reduced. However, new evidence shows that this process could, in fact, be very useful in genebanks.
Case study: Primed and ready

In a study of a temperate woodland plant, common foxglove (Digitalis purpurea), scientists from the Royal Botanic Gardens at Kew in the United Kingdom kept seeds in experimental storage for 14 or 28 days. The seeds were then primed - taken out of storage and rehydrated enough to wake up biochemical pathways within the seed, without actually starting germination, then dehydrated again and placed back into storage. As a result, the seeds showed substantially increased life spans.
The effect of priming is to rejuvenate the seeds. This intriguing result was greatest in the species with shortest-lived seeds. The scientists found that repeated cycles of storage and priming yielded further benefits to longevity. This kind of dehydration-rehydration treatment could be of great interest to genebanks. There are hopes that, used alongside regeneration, processes like this could improve the survival of seeds in storage.
Pause for reflection
Many experiments that have contributed to our knowledge about seed longevity have been carried out under experimental storage conditions (conditions of high temperature and humidity which deliberately speed up aging reactions). Give yourself a few minutes to think about this. How can we be sure that the biological processes are similar, whether germination takes place under experimental storage conditions or actual genebank storage conditions?
Modeling longevity during storage
You have learned so far that although we cannot predict the longevity of an individual seed, when you look at populations of seeds, repeatable patterns start to emerge. You have also learned that moisture content and temperature are important to longevity of seeds in storage. These factors can be analyzed and quantified, in ways that allow us to make mathematical models which help us to predict the longevity of seeds in storage.
In the next section, you will explore a tool that performs this type of calculation. Although we will not be going into the statistical formulae in depth, they rely on the existence of certain viability constants that researchers have divined over the years. These are defined in Table 2 (below).
| Term | Meaning |
| KE | The expected longevity of a population of seeds at 1% moisture content and 0°C. This is a useful concept, because inherent longevity varies between species, and enables us to make comparisons between species.
|
| CW | Another species-specific constant, which describes the effect of making changes to moisture content during storage. This too varies between species, allowing comparisons to be made.
|
| CH and CQ | These two constants together describe the effect of changes in temperature of storage on seed longevity as a quadratic relationship. Unlike KE and CW, these constants have the same ‘universal’ values that do not vary between species.
|
Table 2: seed viability constants
You are now ready to use these viability constants, and other data, and make predictions about seed behavior in storage.
The Seed Information Database

In module 1 you visited the Seed Information Database and saw some of the types of evidence scientists have assembled about seeds. The same online resource also contains a tool that allows you to use this evidence to make predictions about the behavior of seeds in storage.
Activity 2: exploring the Seed Information Database
Allow 10 minutes for this activity.
Now link to the ‘final viability tool’ in the Seed Information Database. It is best to open this link in a new tab or window (right mouse click or long press) to enable you to return to this page easily.
Choose chickpea (Cicer arietinum) from the drop-down list in the ‘Select a preset’ field. This will show you the seed-specific viability constants you discovered in the previous section. Enter the number 6 in the ‘equilibrium moisture content’ field (you will learn more about this in module 4). In the three next fields, enter a typical storage temperature for your own genebank, an initial viability, and a number of days in storage (we suggest you go above 365 days), then press ‘submit’. You should see final viability in the box on the right hand side. What happens if you change the temperature or the time spent in storage?
You might also like to choose another species that you are interested in, and see how the predictions for this species differ from those for chickpea.
Use the note-writing box below to record what you have learned. These notes are for your own development: they will not be shared with course colleagues or moderators.
Please note that these estimates will not always correspond to actual seed behavior in storage. Whether seeds are being stored for food security, the conservation of endangered wild species, or for the future of agricultural research, we are only at the very beginning of understanding the effect of storage on their longevity. This underlines how much genebanks still have to learn about creating the optimum conditions for seeds to survive in storage.
My genebank and me
For your next blog entry, think about all the conditions of drying and storage that go on at your genebank, which could have an impact on longevity and viability. What does the Seed Information Database tell you about their impact on viability of the species you have in your genebank?
Can you think of any other processes in your genebank, which might also affect viability? What could you do to make sure the seeds you send out to users are as viable as they possibly could be?
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Viability testing
One of the most important ways you can secure the conservation of PGRFA in your collection is to carry out regular viability testing. Keeping records of the conditions under which seeds were dried and tight controls on the conditions under which they are stored will help, but regular viability testing is your most reliable guide to when you need to regenerate, and put in a new, rejuvenated stock of seeds.
During the course of viability testing, some seeds will not germinate. It is important to evaluate these non-viable seeds. They can yield useful information about the causes of loss of viability. Are the seeds dead, dormant, quiescent or empty? Are there signs of disease, which requires treatment?
In the next documentary, scientists at IRRI and IITA show how they test the viability of seeds that have been in storage. Even if those seeds were dried and packed correctly prior to storage, and kept under ideal conditions during storage, once they come out of storage there are new considerations that can prevent seeds from meeting the target threshold for germination. As you watch the video, think about how the scientists at IITA and IRRI give their seeds the best possible chances of germinating.

Transcript: Video 2: Monitoring viability
Use the box below to comment on how scientists at IITA and IRRI give seeds the best conditions for germination. You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
Both rice and legumes need to be kept dry during storage, but in order to germinate, they need plenty of water. Scientists at both IITA and IRRI give their seeds the best possible conditions for germination, in terms of water, temperature, humidity and light: the details vary between species. The seeds will not all germinate at once, and those that have not yet germinated are given time to do so. Sometimes, species-specific factors prevent germination: the genebanks take active steps to overcome these. At IITA, Kafayat Falana encourages a legume to germinate by breaking the seed coat. At IRRI, a technician removes the hull of wild rice seeds in order to break dormancy.
Exploring CGIAR’s viability monitoring data
CGIAR Centers have viability records dating back more than thirty years. If you do not work in a CGIAR Center, it is still instructive to look at how these genebanks have managed to maintain the viability of seeds in their collections, and to learn from their records about how different species behave in storage.
Activity 2
Allow 15 minutes for this activity.
Figure 12 (below) shows the viability monitoring data for rice, Oryza sativa in long-term storage (LTS) and medium-term storage (MTS) in two different CGIAR Centers: Africa Rice and IRRI. Take a look at the data: what does it tell you?
Use the note-writing box below to explain what these data tell you about viability of this type of rice in Africa Rice and IRRI.
When you are ready, press 'reveal' to see our comments.
Discussion
IRRI is a long-established Center, which has been keeping records for decades, whereas Africa Rice is newer. This explains why there are more data for IRRI than Africa Rice. Germination rates following medium-term and long-term storage vary, with a few falling below the Genebank Standards’ recommended threshold, but many are above p85. Overall, the data from IRRI shows that high viability has been maintained for many seed lots, over a period of decades, in both medium-term and long-term storage. The results compare well with other studies in the literature.
Not all species and genera are as easy to store as Oryza sativa. Some crops (e.g.: banana) cannot be stored because seeds are not the primary propagation method. Other crops (e.g.: avocado) produce recalcitrant seeds. In future, cryopreservation could offer an interesting conservation opportunity for crops like these. However, even among crops that do produce seeds that can be stored in seed genebanks, there are some whose seeds are more difficult to store than others.
Look at Figure 13, which shows a CGIAR Center’s monitoring data for peanut, Arachis hypogaea in long-term storage (LTS) and medium-term storage (MTS):
Use the note-writing box below to explain what these data tell you about viability of Arachis hypogaea.
When you are ready, press 'reveal' to see our comments:
Discussion
The data for medium-term storage show a lot more variation in viability for this particular type of groundnut than you see in other crops. The results of germination tests vary a lot. Paradoxically, this effect is less obvious in long-term storage of Arachis hypogaea. This shows we cannot be complacent about some crops, even in international genebanks – there is still a lot for us all to learn!
Despite differences between crop species, the CGIAR viability data gives reasons for optimism about the effectiveness of genebank operations:
- Genebank conditions maintain viability well (enough) for most crop species.
- Given predicted and achieved longevity of seeds of the various crop species, regeneration is primarily due to low seed number, often at harvest, rather than loss of viability.
- We need to optimize the quantity of seeds produced and placed into storage to represent an accession, and to base decisions about how many seeds to store on expected demand - if there is any demand.
- For some species, which are relatively short-lived, we may be approaching ‘steady state’ operations; however, for many species, genebanks are still too ‘young’ to be certain of what longevities can be achieved.
- It is important to continue monitoring older seed lots, maintaining previous viability testing intervals, or even shortening them, to get a better understanding of longevity in genebank storage.
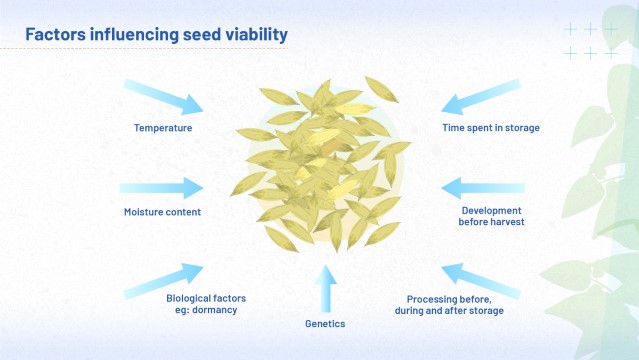
Factors influencing longevity
So far, we have explored how scientists investigate longevity and viability, and considered some of the factors that can affect these qualities. Back in the 20th century, a vast body of evidence was built up to support the idea that the lower the storage temperature, and the lower the moisture content of the seeds, the longer the period of viability for seeds in storage. Other scientists during the last century conducted experiments to investigate the gaseous environment: they built up sufficient evidence for us to assert that for most species, the higher the oxygen pressure, the shorter the period of viability.
More recent experiments confirm that time spent in storage is an important predictor of how a seed lot will perform in germination tests. This issue has become more pressing as genebanks, and their collections, come of age in the 21st century. Meanwhile, discoveries from priming experiments have given us a more nuanced understanding of the impact of moisture content. These experiments have suggested ways in which manipulation of the moisture content of seeds may improve viability. There are other influences: seeds may be immature, dormant, quiescent or empty. You will learn more about these influences later in the course.
Figure 14 summarises the full range of conditions of the storage environment, which can affect the viability and longevity of seeds. You might like to keep a high quality version of the image, so you can refer back to it in future. To do this, first click the 'Maximise' link below the image: to download, right click or control click once the image has opened.
Now that we have considered all the factors that may influence seed longevity, it is time to return to the Genebank Standards for storage, and consider how they might be modified by genebanks, depending on species and context.
The FAO’s Genebank Standards
The storage conditions currently laid out in the FAO’s Genebank Standards reflect current best practice. Having read the previous sections, hopefully you now understand the type of scientific evidence they are based upon. However, they do not differentiate between species. Here are some examples, taken from the Genebank Standards:
4.2.1 All seed samples should be dried to equilibrium in a controlled environment of 5–20°C and 10-25% RH, depending upon species.
4.2.2 After drying, all seed samples need to be sealed in a suitable airtight container for long-term storage; in some instances where collections that need frequent access to seeds or likely to be depleted well before the predicted time for loss in viability, it is then possible to store seeds in non–airtight containers.
4.2.3 Most-original-samples and safety duplicate samples should be stored under long-term conditions (base collections) at a temperature of –18 ± 3°C and 15 ± 3% RH.
4.2.4 For medium-term conditions (active collection), samples should be stored under refrigeration at 5–10°C and 15 ± 3% RH.
POLL: you decide
On the basis of what you have learned so far, do you think the Genebank Standards need to change?
Let’s see what you and your course colleagues think. Please express your opinion about the Genebank Standards by following this link to the poll.
The results of this poll may be discussed at the next live event.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Discussion point: the Genebank Standards
This is the second discussion-based scenario of the course.
Participation in this and the other discussions within the course is essential for you to gain your end-of-module badge and completion certificate. The course organizers will visit the discussion spaces now and then, and will provide feedback and guidance, so please do go back to the discussion space to check the organizers’ feedback.
You should use these discussions to:
- Explore common dilemmas in seed quality management
- Listen to the opinions of other learners and the facilitator
- Come to a collective view on how to proceed when faced with this type of scenario in your own work
Technical tip
By entering the discussions through the ‘Discussion spaces’ menu, clicking the ‘Subscribe to this group’ button at the end of the discussion, and adding your email address, you will receive a notification every time a new message is published in the discussion space.
Discussion space: advice for a genebank manager
A genebank manager has asked you for advice on how to improve the longevity and viability of seeds in their genebank. Bearing in mind what you’ve now learned in module 2, what additional advice you would give, to complement the advice already given in the Genebank Standards? In which areas of the genebank’s operations might this genebank manager start looking for improvements to the longevity of seeds in medium and long-term storage?
Discussion spaces:
As you move through the course, you may wish to return to the discussion spaces to add further posts or to read what other learners have posted.
You can access the discussion spaces from the main menu on the left-hand side of this page.
Key words and concepts

Throughout this course, we offer you quizzes to help you keep track of how much you have learned. We hope you find them enjoyable. The quizzes within modules are not graded, and your results will not be shared with colleagues, but engaging with these quizzes is crucial to your success on this course. It is important to check your answers and read the feedback we have written: this feedback is often the best way to learn. It will help you to develop your own understanding.
Working through the quizzes in every module will build your confidence until by the end of the course, you will be ready to tackle the end-of-course quiz. Unlike the quizzes within modules, this final quiz will count towards your badge and statement of participation.
Have a go at this quiz, testing your understanding of key ideas in module 2.
The Forum
The Forum gives you the opportunity to meet course organizers and moderators and discuss what you have learned so far. It contains important information about your course, and enables you to talk to your colleagues about the ideas that emerge in the modules. It is therefore a good idea to get into the habit of going to the Forum regularly, and not just at the end of a module.
If you have any queries or reflections, there is a designated Q&A strand within the Forum, where you can post your questions. The course organizers will either answer on the Forum, or in the next live event.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Live event
The first live event of the course is scheduled to correspond with this module. It will focus on the content of modules 1 and 2. Look out for emails from the course organizers about when this will happen. It is important to make sure that you get through all the online material for both modules 1 and module 2 before attending the live event.
Please use this space to reflect on the live event. These notes are for your own development and will not be shown to your moderators or colleagues. You will be able to download them at the end of the course.
Summary
You have reached the end of the online learning component of module 2. In this section, we have taken a long, hard look at the science of seed longevity and viability. You have seen how important it is to ensure that seeds in storage are kept under optimal conditions, and that germination tests are carried out regularly. However, a lot can happen to a seed lot before storage begins. In the next online section, module 3, you will discover of what happens to a seed while it develops in the field, how it changes after harvest, and why these events can have far-reaching consequences for genebanks.
Module 2 at a glance:
- Maintaining seed longevity and viability in storage is a crucial aspect of a genebank’s work.
- The viability of seeds in storage is often relatively stable after a short period, then tails off more steeply in a predictable, S-shaped curve.
- The longevity (p50) of a seed lot can be calculated by growing-out a sample of seeds, and reading off the time at which 50% of the sample have lost viability.
- Factors influencing the longevity of seeds in storage include time spent in storage, moisture content, temperature, and processes carried out during storage (e.g.: priming).
- The Genebank Standards give general advice on optimizing conditions in storage; however, there is evidence of differences between species, so that optimal conditions for a particular species may be different from those given in the Genebank Standards.
Useful publications
If you were interested in some of the issues in this module, you might like to download and read these articles, which we have selected for you.
Butler et al. (2009). Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Ann. Bot. 103, pp. 1261–1270.
Available at: https://DOI.org/ 10.1093/ aob/ mcp059.
FAO (2014). Genebank Standards for Genetic Resources for Food and Agriculture. Rev. Ed. Rome.
Available at: https://www.fao.org/ 4/ i3704e/ i3704e.pdf.
FAO (2022). Practical guide for the application of the Genebank Standards for Plant Genetic Resources for Food and Agriculture: conservation in seed genebanks. Commission on Genetic Resources for Food and Agriculture, Rome.
Available at: https://doi.org/ 10.4060/ cc0021en.
Hay, F.R., Whitehouse, K.J., Ellis, R.H., Sackville Hamilton, N.R., Lusty, C., Ndjiondjop, M.N., Tia, D., Wenzl, P., Santos, L.G., Yazbek, M., Azevedo, V.C.R., Peerzada, O.H., Abberton, M., Oyatomi, O., de Guzman, F., Capilit, G., Muchugi, A. and Kinyanjui, Z. (2021). CGIAR genebank viability data reveal inconsistencies in seed collection management. Global Food Security, 30, 100557.
Available at: https://doi.org/ 10.1016/ j.gfs.2021.100557.
Whitehouse, K.J., Hay, F.R. and Lusty, C. (2020). Why seed physiology is important for genebanking. Plants, 9(5) p 584.
Available at: https://doi.org/ 10.3390/ plants9050584.
Yong-Bi, F., Zaheer, A., Diederichsen, A. (2015). Towards a better monitoring of seed ageing under ex situ seed conservation.Conservation Physiology. 015; 3(1).
Available at: https://doi.org/ 10.1093/ conphys/ cov026.