Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 22 February 2026, 3:34 AM
Module 3: Harvesting and processing
Introduction
Welcome to the third module of this online course. In module 2, you learned about some key factors influencing longevity and viability of seeds while in genebank storage. However, the influences on a seed’s ability to germinate do not start at the moment it goes into storage.
In this module, we will take you on a journey through the early stages of a seed’s life. You will discover natural processes, such as seed development, climate, irrigation, and the plant’s own seed dispersal mechanisms. All of these can affect the longevity of seeds in your care, and you can exploit or mitigate some of these natural processes. By knowing how much to irrigate, when to harvest, and how to manage the processing that goes on in the lab between harvesting and storage, you can make a difference to your genebank’s success in conserving biodiversity.
By the end of this module, you will be in a good position to apply what you have learned to your own work. We will give you the opportunity to read about the science of this in more depth in our ‘Useful publications’ section.
By the end of this module, you should be able to:
- Describe the main stages of maturation in seeds prior to harvesting.
- Give examples of the effect of different harvesting strategies on seed longevity.
- List processes in a genebank that happen after harvesting, which can have an impact on seed longevity and viability.
- Discuss what can be done by genebanks after harvesting, and to what extent this can compensate for what has happened to the seeds before harvesting.
- Explain how genebanks can improve their practice based on research evidence.
- Evaluate the procedures in your own workflow.
Overview of genebank processes
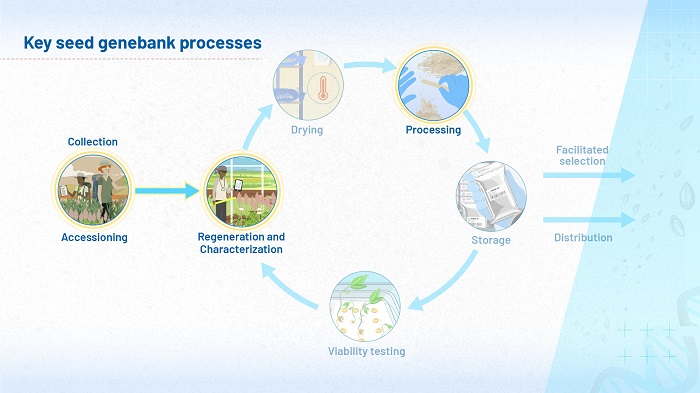
Figure 1 (above) highlights the stages of a genebank’s operations you will consider in module 3. Here, you will shift your focus to the science underlying regeneration, characterization and processing. Parts of this module are also relevant to the collection of brand new accessions, being placed in a genebank for the first time.
During seed regeneration (which in some contexts is referred to as ‘rejuvenation’ or ‘multiplication’), there is a period when the crop is growing in the field. On the parent plant, seeds develop from tiny globules into mature seeds, capable of germinating into new plants. They must be harvested at exactly the right level of maturity. After harvesting comes threshing, cleaning and processing after the material enters the genebank. All of these can affect a seed’s longevity when it enters storage.
In this module, we will take a deep dive into the biological mechanisms that occur while seeds mature, and that continue during the human-imposed processes that are conducted in a genebank, before the seeds go into storage.
Some definitions
Fertilization, which happens after pollination, is the fusion of the male reproductive cell (pollen) with the female reproductive cell (ovum) to form a zygote.
The male and female reproductive cells are usually haploid – they each have one copy of each chromosome. Once they combine, the resulting zygote is usually diploid - it has two copies of each chromosome.
The process of seed development is ultimately regulated by genetic controls, which can switch developmental processes on and off.
As a seed matures, seed filling is the process of transferring carbohydrates, lipids, proteins, and other components into the developing seed.
The stage of mass maturity is when maximum seed dry matter has accumulated, at the end of the seed filling phase.
During seed dispersal, seeds are spread or transported away from the parent plant, giving them the chance to germinate and grow into new plants.
The embryo of a seed is the tiny, furled up living baby plant, which in future may become a full adult plant.
Attached to the embryo are the cotyledons, stores of nutrient that protect and nourish the growing embryo, and become the new plant’s first leaves.
Seeds of some species, such as cereals, have most of their food storage reserves in tissue called the endosperm. Other species, such as legumes, have food storage in their cotyledons, while yet other species have a combination of both these food storage systems.
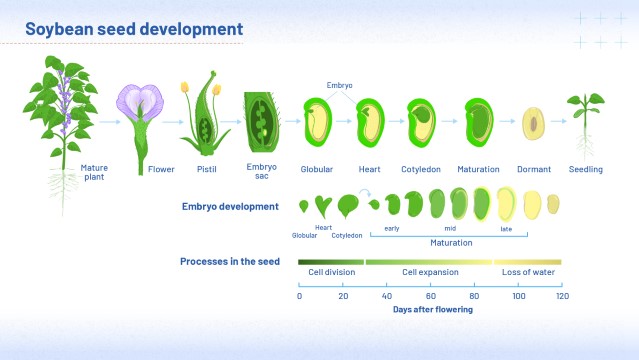
Development of a soybean seed
Seeds are essential to plants’ survival: they protect and nourish the developing embryo that will eventually become the next-generation plant. In legumes, the seed has two cotyledons, which become more defined as the seed develops.
Figure 2 (below) shows you the developmental process of soybean, a typical legume. You might like to view and download this image at high quality. To do this, first click the 'Maximise' link below the image: to download, right click or control click once the image has opened.
Our story starts with a flower on the parent soybean plant, dusted with pollen grains. Making its way to the female reproductive cells or ‘gametes’ inside the flower, a tube develops from each pollen grain, allowing male gametes to travel to the base of the flower. This is where fertilization occurs.
After fertilization, the first diploid cells to appear are a tiny globular mass of dividing cells, which develop into a heart-shaped embryo. It takes some days for the cotyledons to become distinguishable, but once they do, they grow larger and larger, eventually becoming bigger than the embryo. Inside the cotyledons, proteins, carbohydrates and lipids are being built up in a process called ‘seed filling’. Once the seed has matured, water is expelled. As the seed continues to lose water, it shrinks a little, so that by the time it is ready for harvest, the cotyledons are more compact than they had been earlier in the process.
In the rest of this section, you will discover more about what happens during seed development, and why this matters so much to genebanks.
Pre-storage factors
Everything that happens to seeds before they go into storage can affect their longevity. One reason for seeds failing to germinate when they come out of storage could be that they were immature when they first arrived at the genebank. Figure 3 (below) shows the changes that normally take place when seeds mature on the parent plant, while they are still in the field.
A very young seed is effectively a tiny, globular mass of cells in a bag of water. Figure 3 shows the changes that follow, as it develops into a mature seed. The brown curve shows how dry weight increases, as the seed fills with carbohydrates, lipids, proteins and so on. The blue curve shows how moisture content decreases over the same period.
The large molecules build up until the point we call ‘mass maturity’, where the brown curve flattens. At this point, there ceases to be a vascular connection between the parent plant and the developing seed. No more large molecules can enter the seed. Whereas the blue curve shows how moisture content continues to decline steeply, even after mass maturity.
It should come as no great surprise that if you harvest immature seeds, you’ll have a hard job convincing them to germinate. However, as a genebank scientist, the germination of freshly harvested seeds is not the only thing you need to think about. You also need to be sure your seeds will germinate after being dried and stored for many years at low temperature!
Figure 4 (above) superimposes the results of germination tests over the development graph shown in Figure 3. The green curve shows how germination improves for fresh seeds the longer after flowering they are harvested. These fresh seeds are already close to their maximum capacity for germination when they reach mass maturity.
By contrast, the orange curve shows what happens when the seeds are air-dried before attempting to germinate them. For these seeds, harvesting at mass maturity does not yield optimal germination rates – their longevity will improve if you wait and harvest later than mass maturity.
The purple plot shows longevity when seeds are both dried and kept in storage: these seeds are even less likely to germinate if harvested at mass maturity. They only come into their own very late in the maturing process, at around the time when the wild plant naturally disperses its seeds. This is less common in domesticated crops, where shattering resistance is a common trait, meaning that the seeds remain on the plant until they are harvested.
Activity 1
Allow 5 minutes for this activity
Take another look at Figure 4 above. Based on this evidence, at what point would you advise a colleague to harvest the seeds of this plant, in order to maximize longevity in storage? Use the text box to write down your prediction.
When you are ready, press 'reveal' to see our comments.
Discussion
Your colleague would do well to harvest these seeds after the vascular connection with the parent plant has severed, and the moisture content has reduced as much as environmental conditions in the field permit. This is why gene bankers are advised to harvest wild species as close as possible to natural seed dispersal, because that is when their longevity in storage is likely to be at its greatest.
Seed dispersal mechanisms
In the wild, plants have developed various seed dispersal strategies to ensure the survival of their seeds. They come into play after seeds have matured, and can give the seeds a competitive advantage. In such plants, seed longevity continues to improve as the seeds dry down to equilibrium with ambient conditions. Viability does not peak until very close to seed dispersal. Let’s look at a couple of examples.
Case study: bursting with viability

In wild relatives of crop species such as peas, the natural seed dispersal mechanism is what biologists call ‘explosive’. After maturing, the pod splits open suddenly so that the seeds burst out, and are propelled far enough to land some distance away from the parent plant. This helps young seedlings to survive in situ, by reducing competition with the parent plant.
Crop species descended from plants with this dispersal mechanism retain the tendency for longevity to improve until very late in seed development. While the weight of the seeds stabilizes, they are still drying out in preparation for dispersal. This lower moisture content has a beneficial effect on longevity in storage, which keeps improving until very close to the time of dispersal. This is why, in many crops, it is a good idea to harvest as close to the point when the plant naturally disperses its seeds as possible.
Explosiveness can be a challenge in a wide variety of crops. For instance, some wild varieties of rice are highly shattering. They may release seeds before the seeds are fully mature, while their moisture content is still high. By harvesting too soon, the seeds will be immature, and not ready for storage. But if you wait too long before harvesting, the seeds you have carefully cultivated could disperse and be lost to your genebank. Gathering seeds from the ground is not good practice. CGIAR genebanks have developed a range of strategies to overcome these challenges, which you will learn about next.
Deciding when to harvest

CGIAR scientists at the International Rice Research Institute (IRRI) conducted a series of experiments to investigate the impact of seed maturity at harvest on longevity in storage. In one set of experiments, twenty Oryza sativa accessions were harvested at 24, 31, 38 and 45 days after flowering. They were then dried in the genebank’s drying room, grown-out, and their longevity (p50) calculated. In many cases, the greatest longevity was in seeds that had been harvested at 45 days, although some peaked at 38 days and declined after that. Figure 5 shows what a difference a delay can make:
By allowing seeds to mature for as long as possible on the parent plant, it is possible to improve their longevity in storage. But the seed’s maturation process is not the only pre-storage factor that can have an effect on longevity. The weather can make a difference too.
Conditions in the field
Imagine you had one lot of a rice cultivar growing in a cool climate (20-28°C), and another lot of the same cultivar growing in a warmer climate (24-32°C). What difference would the different climates and temperatures make to the seeds’ longevity in storage? A research team from the University of Reading did some experiments to find out.
Figure 6 (below) shows the behavior of the seeds of Japonica rice, a type of rice normally grown in temperate environments. The square readings are for seeds grown in a warmer climate, the round ones are for seeds grown in cooler conditions. You can see that at both temperatures, the moisture content drops and the mass initially increases, then stabilizes at mass maturity, as you’d expect from the generalized patterns you saw in Figure 3 on the page entitled ‘Pre-storage factors’.
However, something else must be going on: the researchers discovered differences in longevity associated with the two growing conditions after those seeds have been harvested, dried and stored. The seeds grown in the cooler climate achieved higher longevity than those grown under warmer conditions. In other words, for Japonica rice, the quality of seeds grown under certain climatic conditions is better than the quality of seeds grown under other conditions.
If temperature during development can affect how seeds behave in storage, you might wonder if the presence or absence of rain (or indeed irrigation) could be another factor. Another team from Reading set out to investigate, this time using Brassica seeds with different irrigation regimes, to simulate the effect of drought.
Figure 7 (above) shows three samples of Brassica. The red triangles at the base show the time of mass maturity. The graph with round points shows what happens when the parent plant was continuously irrigated. For the graph with triangular points, irrigation was stopped 16 days after pollination, and for the graph with square points, irrigation was stopped 24 days after pollination, imitating what might happen during a severe drought.
Activity 2
Allow 5 minutes for this activity
Take a look at Figure 7 and decide what changes you can see, as a result of different lengths of drought at the end of seed development. Use the text box to write your thoughts.
When you are ready, press 'reveal' to see our comments.
Discussion
Stopping irrigation before harvest had an impact on how quickly the seeds developed: the earlier the irrigation stopped, the quicker they reached mass maturity. In the continuous irrigation group, there is a small dropping-off of potential longevity the later you harvest after mass maturity, whereas in the two experimental conditions, where an artificial drought was imposed, this decline in potential longevity is steeper.
Genebankers’ perspectives
Let’s revisit the two genebanks, IITA and IRRI. The next documentary explores what goes on while seeds develop in the field, and how seed scientists and genebank managers decide on the best time to harvest. The video tells the story of a seed’s early life, from flowering to threshing. By tracking this development carefully, the scientists can make a difference to the physiological quality of seeds when they enter the genebank.
As you watch, think about how the genebanks harness seeds’ developmental processes to maximize their longevity in storage.

Transcript: Video 1: from field to genebank
Please use the box below to write how understanding the biological processes helps the IITA and IRRI scientists to ensure the viability of seeds in their genebank. How could these measures be adopted in your own genebank? You should spend up to ten minutes on this. If your reflections on the video raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
If seeds are to maintain their viability during storage, they must first be at the right level of maturity when they arrive at the genebank. Fiona Hay and Olaniyi Oyotomi explain the developmental processes leading up to this optimum level of maturity. It takes a predictable number of days to complete the transformation, so genebankers keep careful records of the number of days after flowering. The parent plant gives visual signs as well. Sola Owoborode, Andres Godwin Sajise, Oladepo Adebowale and Olubiyi Adeyemi demonstrate the clues to look out for. Weather permitting, it is best to wait until the final stage of seed development, during which time seeds dry down to equilibrium with the surrounding air.
Conditions in the genebank

Until a consignment of seeds is packed for storage in a genebank’s active and base collections (or Svalbard, or another safety duplicate location), time is of the essence. Delays between the seeds’ arrival at the genebank and their going into storage can make a big difference to longevity and viability. So can differences in humidity and temperature while they wait to be processed.
Although conditions in the field and at harvest are not always under the control of a genebank, there is tantalizing evidence that, by making small changes to the conditions and timescale before they are processed, it may be possible for a genebank to compensate for less-than ideal growing or harvesting conditions. Let’s start with humidity and temperature.
Humidity and temperature
You have already learned that seeds arriving at a genebank may be immature, or may contain more moisture than is optimal for longevity in storage. This may happen if weather conditions in the field are poor, or if the seed’s natural dispersal mechanisms make it expedient to harvest before the seeds shatter and become spread over the ground, therefore lost to science. In this situation, once the immature seeds arrive at the genebank, they can mature under safe conditions in the drying rooms.
Many genebanks dry seeds according to the Genebank Standards: at temperatures between 10 and 25°C, and humidity between 10 and 15%. This contrasts with traditional practices of farmers in countries with hot, dry climates, who leave their crops out to dry in the sun, where temperatures can be around 40°C. The traditional method is low cost and tried and tested, although from a genebank’s point of view, it has limitations: prolonged exposure to the sun can damage seeds and cause a reduction in viability, and drying outside can be impractical during the rainy season.
So which of these two approaches would you expect to be more effective – drying at high temperature, like traditional farmers, or at temperatures that are prescribed in the Genebank Standards? Scientists at IRRI set out to find out.

In the case of rice, it seems the prescription in the Genebank Standards may not be optimal for producing seeds with high viability and longevity. The IRRI researchers tested the viability of samples of Oryza sativa that had been dried according to the Genebank Standards, and compared their survival with the survival of other samples, initially dried for two days in a batch-dryer at 40–45°C, before being transferred to the drying room for the rest of the drying period. Intriguingly, initial drying at high temperatures, perhaps more akin to ideal conditions when the seeds are maturing in the field, or traditional roadside drying, had a lot to offer.
Figure 8 (below) shows the improvements in longevity that resulted from initial drying at a higher temperature. There were no negative effects, and in many cases, a significant increase in longevity (p50).
Activity 3
Allow 5 minutes for this activity
Look at Figure 8 (above). What are the improvements in longevity when the seeds were initially dried at higher temperature?
When you are ready, press 'reveal' to see our comments.
Discussion
There are no negative effects in any of these accessions, and in some cases, there are very significant positive ones. Where the bar chart reaches one hundred percent (for instance accessions 117264 and 117265) this means a doubling of p50, and in one case (accession 117274), you can see that the expected longevity was more than trebled.
Dried to perfection
The benefit from initial drying at high temperature is not unique to cultivated varieties of Oryza. Figure 9 (below) shows the results for two wild relatives. The two wild species remained viable for longest when they had initially been dried at 45°C (the blue curve) before a second stage of drying at temperatures of around 15°C. They remained viable for less time when they had only been dried at 15°C (the black curve) throughout the drying process. Even seeds that were initially dried at a sweltering 60°C (the pink curve) performed slightly better in germination tests than the seeds grown according to Genebank Standards (the black curve).
These findings suggest that for some species, longevity in storage can be improved by adapting the conditions under which the seeds are dried. These results were compelling enough to convince IRRI to change their procedures. Instead of drying rice according to the Genebank Standards, IRRI now carry out two-stage drying, where seeds are initially dried at 30°C for a couple of days, then finished under conditions the Genebank Standards recommend: 15% humidity and 15°C. This has allowed IRRI to make demonstrable improvements to seed viability.
There is evidence that other species may benefit from drying at different temperatures too. Scientists at the Institute for Tropical Agriculture (IITA) in Nigeria found similar improvements in soya beans and Bambara groundnuts. Although there is a lot we still don’t know, it is likely that if seeds have had to be harvested early, for instance due to poor weather, drying them under conditions that simulate natural conditions in the field during a good year can compensate for losses in viability as a result of being harvested prematurely.
Pause for reflection
You have discovered that genebanks can improve longevities of seeds in their care. Give yourself five minutes to think about whether, if genebanks were able to implement better and better conditions, the improvements in longevity could continue indefinitely? Are there limits to these improvements?
Time between harvest and storage
So far, you have learned the danger of waiting too long to harvest - we don’t want to allow seeds to disperse and be lost. You have learned that seeds should not go into storage when they are immature, but it is also important that they also should not go into storage when they are too old. Watch the animation, which is based on data about the fate of seed lots regenerated by a genebank in 1990. As you watch, think about what was happening to the longevity of these seeds as time went by.
Transcript: Video 2: processing time and viability
In 1990, a fresh accession of seeds was checked in to a genebank.
Some of these seeds were processed in less than 6 months.
Some were processed in less than a year.
Others were left between one and two years before they were dried, cleaned, packed and placed into storage.
What difference did this delay make to their long-term viability once they had gone into storage?
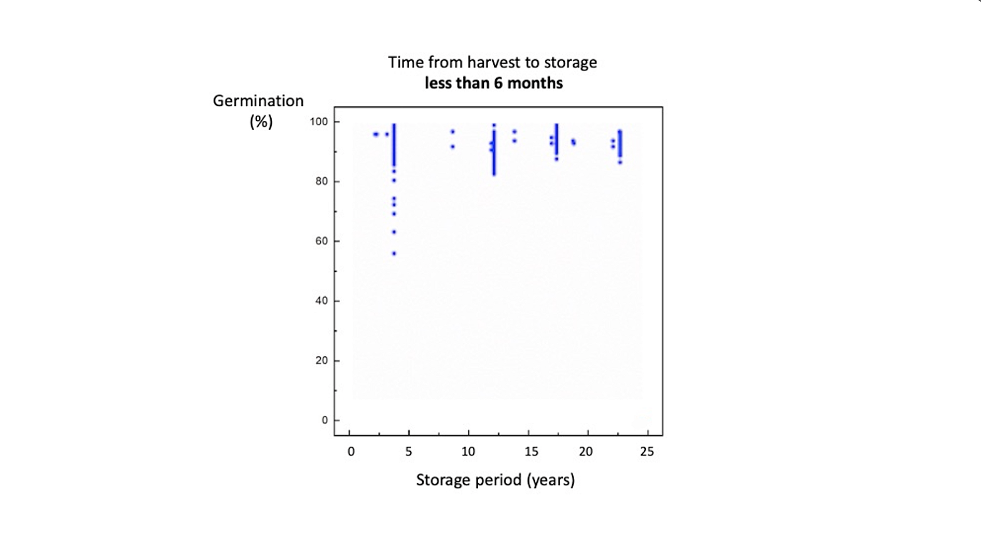
Let’s start with the seeds that were processed within six months.
After 3 years, the results of germination tests ranged from fifty to 100%. The lower results are probably because some of the seeds were dormant.
After 12 years, this situation has improved: most of the dormancy has worn off.
After 16 years, the results are satisfactory - about 85 to 98%.
Even after 20 years, viability has not declined a lot.
What about the seed lots which went into storage after 6 months to one year?
Once again, the initial results could perhaps be explained by dormancy.
But by 10 years, dormancy can’t explain why the lowest viability has dropped to a measly 30%.
And after 16 years, when you’d expect dormancy to have passed, there is still a wide spread of viability.
At 20 years, although some seeds are highly viable, the results are more patchy than for the seeds that were fresh when they first went into storage.
Much the same is apparent for seeds that waited between one and two years before they went into storage.
There’s a noticeable tendency for these seeds to perform less consistently in germination tests compared to the viabilities of the seed lots that had been placed into storage in less than 6 months.
These results suggest that when freshly harvested seeds arrive at your genebank, there’s no time to waste.
Please use the box to note down what happened to seed lots put into storage after 0-6 months, 6-12 months, and 1-2 years. What implications does this have for your own genebank? You should spend up to ten minutes on this. If your reflections raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
Seed lots put into storage up to six months after harvest did not, at first, germinate as strongly as perhaps you might expect. This may be because some seeds were still dormant: a little longer in the drying room might have broken this dormancy. Nevertheless, these seeds show strong viability over the long term. Seed lots put into storage within a year of being harvested were able to germinate vigorously, even after more than twenty years of storage. By contrast, in seed lots which had to wait years before going into storage, there is a noticeable tendency to perform less well in germination tests when they came out of storage. These data show that delays in processing seeds before storage can compromise longevity and viability.
My genebank and me
For your next blog entry, think about what happens in your genebank between harvesting and storage.
Are there delays? If so, can you think of any ways you could reduce those delays, and therefore safeguard viability? What could you do in your personal practice, to make sure your newly regenerated seeds remain as viable as possible once they are in storage? If you would like to share your ideas with other participants and the moderators of the course, write a post in the Forum.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Beating the clock
This module should have convinced you how important it is to protect seeds while they are being processed between harvest and storage. Genebanks can control the temperature and humidity of the areas where seeds are processed: this does a lot to reduce aging reactions. But there are a lot of processes to get through, so perhaps the biggest challenge is controlling the amount of time it takes to process the seeds.
Watch this video, which shows the number of processes a genebank must carry out before seeds are ready for storage. Scientists at IRRI and IITA demonstrate these processes, while Fiona Hay and Olaniyi Oyatomi discuss some steps genebanks can take to ensure all processes are carried out efficiently. As you watch, think about how the scientists ensure the seeds’ passage through the genebank is as swift as it can be.

Transcript: Video 3: beating the clock
Please use the box to list some of the processes mentioned in the video. How important are they? What facilities do genebanks need to get through them? You should spend up to ten minutes on this. If your reflections raise any questions, please post them on the Forum, where the course moderators will be able to help you.
When you are ready, press 'reveal' to see our comments.
Discussion
You might have listed drying, cleaning, quality control, phytosanitary controls, counting or packing. They are very important. In fact, it is hard to see how a genebank could fulfil its aims without them! If a genebank compromises on sorting, the genetic purity of their collection could be at stake. If immature seeds are allowed to go into storage, they are unlikely to germinate. Likewise, there is no point in storing seeds that are too unhealthy to pass phytosanitary tests. Although there are high-tech solutions, for many genebanks, affordable technologies like barcoding and good data management help to ensure seeds are processed efficiently.
Genetic basis of longevity
Within any species, there is variation in seed longevity, even within closely-related germplasm. This can provide vital information for seed genebanks about the molecular basis of this trait. Consider the development of a soybean seed, which you learned about at the beginning of this module. Every protein stored in the developing cotyledons is encoded by a gene; every process is switched on and off by genetic mechanisms, such as DNA methylation. These genetic controls continue as long as the seed survives. By deepening our understanding of these processes, there is potential to make further improvements in seed longevity.
A team at IRRI set out to determine the relative seed longevity of various accessions of Indica rice (Oryza sativa Indica), harvested at intervals between four and five weeks after flowering. The accessions were equilibrated at 10.9% relative humidity and 45°C. Samples were taken at regular intervals, and germination tests were carried out, to reveal the loss of viability during experimental storage. As you might expect, longevity tended to be higher in samples with lower harvest moisture content, and less in samples with higher harvest moisture content.
The study also gave glimpses of a possible genetic basis for longevity. In a genome-wide analysis, eight major loci were identified as potentially having some control over seed longevity. Located on chromosomes 1, 3, 4, 9 and 11, they were shown to enhance p50 by ratios of 0.22–1.86. Comparing these findings with existing knowledge about the rice genome suggested that the mechanisms conferring high seed longevity might be related to DNA repair and transcription, sugar metabolism, reactive oxygen species scavenging and embryonic and/or root development.
POLL: you decide
On the basis of what you have learned so far, how much do you think advances in genomics can improve seed longevity?
Let’s see what you and your course colleagues think. Please express your opinion by following this link to the poll.
The results of this poll will be shared with your course colleagues.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Investigating variations in longevity
Genebanks rely on the ability of seeds to go into an induced latent state when they are dried and cooled. Therefore in genebank storage, seeds must be stored at low temperatures and low moisture content to prevent them from aging.
By contrast, scientists investigating seed longevity deliberately place seeds under conditions that will accelerate the aging process. This allows the scientists to explore processes that normally take decades or even centuries, within a realistic timeframe for a scientific experiment. They subject seeds to conditions of high temperature and humidity, which are described variously as ‘experimental storage’ or ‘accelerated aging’.
Figure 10 (below) shows results from the IRRI experiment described on the previous page. The box covers the 25th to 75th percentiles. The green line across the box is the median; the red line is the mean longevity. The whiskers show the 5th and 95th percentile.
These data show that there is a lot of variation in longevity, even within one subspecies, Oryza sativa Indica. The mean and median p50 are less than half the maximum longevity shown by a few exceptional individuals, and the box for the 25th to 75th percentiles is somewhat skewed towards the minimum longevity, suggesting there might be scope for improvement.
By working with just one variant of rice, there should be fewer alleles to consider, rather than having to worry about the entire genome of the whole species. However, the variation even within this single variety group of rice underlines how much there is to investigate about the genetic basis of seed longevity.
The Seed Information Database
The photograph below places 20 varieties of rice in rows reflecting the landscapes where they are grown - from lowland paddies to sloping uplands. It shows some of the diversity within this crop that is visible just by looking at the seeds. In fact, Asian cultivated rice is divided into different ‘variety groups’ based on their genetic relatedness. Indica rice (at the top) is one of those variety groups, and the previous study focused on accessions from within this variety group. Let’s use the Seed Information Database to model the behaviour of Indica rice in storage.

Now go back to the viability calculator of the Seed Information Database. Look up Indica rice (Oryza sativa Indica). Can you model the behavior of Indica rice under these conditions: 12% equilibrium moisture content at 45°C over a period of 20 days? What happens to final viability when you change the temperature, equilibrium moisture content, or number of days? How does the behavior of Indica rice differ from the behavior of Japonica rice, under similar conditions?
Use the text box below to record what you have learned. These notes are for your own development: they will not be shared with course colleagues or moderators.
The FAO’s Genebank Standards
The conditions for acquisition currently laid out in the FAO’s Genebank Standards recognize the need to avoid conservation of immature seeds, seeds that have been exposed to the elements for too long, and to ensure that seeds are handled carefully after collection and before they are transferred to controlled conditions.
- 4.1.2 Seed collecting should be made as close as possible to the time of maturation and prior to natural seed dispersal, avoiding potential genetic contamination, to ensure maximum seed quality.
- 4.1.3 To maximize seed quality, the period between seed collecting and transfer to a controlled drying environment should be within three to five days, or as short as possible, bearing in mind that seeds should not be exposed to high temperatures and intense light, and that some species may have immature seeds that require time after harvest to achieve embryo maturation.
- 4.1.5 The minimum number of plants from which seeds should be collected is between 30 to 60 plants, depending on the breeding system of the target species.
Discussion point: how well are genebanks doing?
This is the third discussion point of the course.
Participation in this and the other discussions within the course is essential for you to gain your end-of-module badge and completion certificate. The course organizers will visit the discussion spaces now and then, and provide feedback and guidance, so please do go back to the discussion space to check the organizers’ feedback.
You should use these discussions to:
- Explore common dilemmas in seed quality management
- Listen to the opinions of other learners and the facilitator
- Come to a collective view on how to proceed when faced with this type of scenario in your own work
Technical tip
By entering the discussions through the ‘Discussion spaces’ menu, clicking the ‘Subscribe to this group’ button at the end of the discussion, and adding your email address, you will receive a notification every time a new message is published in the discussion space.
Discussion space: driving progress
In module 3, you have learned about improvements some genebanks were able to make to the longevity of certain crop species, as a result of scientific investigations. It is important to emphasize that for most crop species, genebanks are doing a good-enough job in conserving diversity. What, then, are the challenges genebanks face in their attempts to improve their processes still further? How might they go about designing incremental improvements?
What measures can genebanks take to improve on their record?
Discussion spaces:
As you move through the course, you may wish to return to the discussion spaces to add further posts or to read what other learners have posted.
You can access the discussion spaces from the main menu on the left-hand side of this page.
Key words and concepts

Throughout this course, we offer you quizzes to help you keep track of how much you have learned. We hope you find them enjoyable. The quizzes within modules are not graded, and your results will not be shared with colleagues, but engaging with these quizzes is crucial. It is important to check your answers and read the feedback we have written: this feedback is often the best way to learn. It will help you to develop your own understanding.
Working through the quizzes in every module will build your confidence until by the end of the course, you will be ready to tackle the end-of-course quiz. Unlike the quizzes within modules, this final quiz will count towards your badge and statement of participation.
Have a go at this quiz, testing your understanding of key ideas in module 3.
The Forum
Throughout this course, the Forum gives you the opportunity to meet course organizers, ask them questions, and discuss what you have learned so far. The Forum is also the place where you can see other students’ questions and comments, and the experts’ responses to them, so it is a good idea to get into the habit of going to the Forum regularly, using the menu on the left hand side.
A live event will accompany this and the next module. Look out for emails from the course organizers about when this will happen. It is important to make sure that you get through the online material for both modules (modules 3 and 4) before attending the live event.
Technical tip
We recommend you open this in a new tab or window (right mouse click or long press) to enable you to easily return to this page.
Summary
You have reached the end of module 3. In this module you have studied how seeds develop in the field, and how this development can be influenced by external factors such as temperature, climate, humidity and the passage of time.
Once seeds have entered a genebank, you have discovered how drying at different temperatures can help improve their longevity, and how important it is to make sure delays in processing are kept to a minimum. You have glimpsed some of the experiments that are starting to shed light on the genetic basis of seed longevity.
In the next online learning section, module 4, you will shift your attention to water activity in seeds, and discover what happens inside seeds when they are dried.
Module 3 at a glance:
- The longevity and viability of seeds is affected by environmental conditions while the seeds are developing in the field.
- Seeds continue to lose water after they have achieved mass maturity, and this too can affect their longevity and viability.
- When harvested too early, seeds still have high moisture content, which is associated with unacceptably high rates of decline in viability during storage.
- It is therefore important to harvest as close as possible to the time when natural dispersal occurs, and when seeds have dried naturally on the parent plant in situ.
- Where seeds have not dried in situ, initial drying at high temperature (eg: 30°C) followed by drying according to the Genebank Standards can improve the longevity of seeds.
- Whatever happens to seeds after they enter the genebank and before they are placed in storage can also affect longevity and viability.
Useful publications
If you were interested in some of the issues in this module, you might like to download and read these articles, which we have selected for you.
Colville, L., Pritchard, H. (2019). Seed life span and food security. New Phytologist, 224 (2), pp. 557-562.
Available at: https://doi.org/ 10.1111/ nph.16006.
Ellis, R., Hong, T, Jackson, M., (1993). Seed production environment, time of harvest and the potential longevity of seeds of three cultivars of rice (Oryza sativa L.) Annals of Botany, 72, pp. 583–590.
Available at: https://doi.org/ 10.1006/ anbo.1993.1148.
FAO (2014). Genebank Standards for Genetic Resources for Food and Agriculture. Rev. Ed. Rome.
Available at: https://www.fao.org/ 4/ i3704e/ i3704e.pdf.
FAO (2022). Practical guide for the application of the Genebank Standards for Plant Genetic Resources for Food and Agriculture: conservation in seed genebanks. Commission on Genetic Resources for Food and Agriculture, Rome.
Available at: https://doi.org/ 10.4060/ cc0021en.
Hay, F. R., Whitehouse, K. J. (2017). Rethinking the approach to viability monitoring in genebanks. Conservation Physiology, 5 (1).
Available at: https://doi.org/ 10.1093/ conphys/ cox009.
Lee, J. S., Velasco-Punzalan, M., Pacleb, M., Valdez, R., Kretzschmar, T., McNally, K. L., Ismail, A. M. (2019). Variation in seed longevity among diverse Indica rice varieties. Annals of Botany, 124, pp. 447-460.
Available at: https://pmc.ncbi.nlm.nih.gov/ articles/ PMC6798842/
.
Sinniah, U. R., Ellis, R. H., John, P. (1998). Irrigation and seed quality development in rapid-cycling Brassica: seed germination and longevity. Annals of Botany, 82, pp. 309-314.
Available at: https://doi.org/ 10.1006/ anbo.1998.0738.
Timple, S. E., Hay, F. R. (2018). High-temperature drying of seeds of wild Oryza species intended for long-term storage. Seed Science and Technology, 46, 1, pp. 107-112.
Available at: https://doi.org/ 10.15258/ sst.2018.46.1.10.
Whitehouse, K. J., Hay, F. R, Ellis, R. H. (2015). Increases in the longevity of desiccation-phase developing rice seeds: response to high-temperature drying depends on harvest moisture content. Annals of Botany, 116(2): pp. 247–259.
Available at: https://doi.org/ 10.1093/ aob/ mcv091.