Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 23 November 2025, 3:32 AM
The health and economic burden of AMR
Introduction
This course introduces the concepts and methods for estimating the impact of bacterial antimicrobial resistance (AMR) on populations. AMR has a significant global impact on public health and economies, for example by increasing illness (morbidity) and death (mortality), placing a burden on healthcare systems and economic productivity.
This course provides an overview of the burden of disease that bacterial AMR is responsible for, including both morbidity and mortality, and introduces the economics of bacterial AMR. It defines ‘burden’ as an epidemiological term and introduces key epidemiological concepts essential to understanding bacterial AMR and its burden, covers methodologies for measuring AMR burden and considers their strengths and limitations. Additionally, it outlines the economic consequences of AMR on healthcare systems, communities and economies, and explains how cost-effectiveness analysis can guide policy decisions to mitigate bacterial AMR. This course will not cover drug-resistant tuberculosis, HIV or malaria. The term ‘AMR’ will refer to bacterial AMR throughout unless otherwise specified.
This course requires basic knowledge of definitions and sources of data related to AMR and how it can be measured. If you are unfamiliar with these concepts or would like a reminder, you might want to revisit Sections 1–4 of the Fundamentals of data for AMR course before starting this course. If you haven’t done so already, it might also be helpful for you to review the course Introducing antimicrobial resistance to make sure you understand what
After completing this course, you will be able to:
- define key epidemiological terminologies and concepts related to the burden of bacterial AMR
- explain the rationale and value of assessing the burden of disease for AMR
- demonstrate an understanding of the metrics and indicators that are commonly used to describe the burden of AMR
- outline how data collected and analysed using available methodologies for estimating the burden of AMR can be interpreted, highlighting the strengths and limitations of each methodology
- reflect on burden of disease data related to AMR in your settings (including community, hospital, country, and WHO region) and at a global level
- demonstrate an awareness of the direct and indirect economic consequences of AMR and antimicrobial use on healthcare systems, individuals, communities and economies, considering both short- and long-term perspectives
- outline the benefits and costs of One Health strategies to mitigate the effects of AMR
- explain how economic analysis can inform AMR-related policy decisions by identifying interventions that provide the greatest benefit relative to their costs.
In order to achieve your digital badge and Statement of Participation for this course, you must:
- click on every page of the course
- pass the end-of-course quiz
- complete the course satisfaction survey.
The quiz allows up to three attempts at each question. A passing grade is 50% or more.
When you have successfully achieved the completion criteria listed above you will receive an email notification that your badge and Statement of Participation have been awarded. (Please note that it can take up to 24 hours for these to be issued.)
Activity 1: Assessing your skills and knowledge
Before you begin this course, you should take a moment to think about the learning outcomes and how confident you feel about your knowledge and skills in these areas. Don’t worry if you do not feel very confident in some skills – they may be areas that you are hoping to develop by studying these courses.
Now use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
This is for you to reflect on your own knowledge and skills you already have.
1 Key epidemiological terminologies and concepts related to burden of bacterial AMR
This course explores the
This encompasses both health outcomes – such as illness (
Understanding the scale and nature of these burdens is essential for designing effective, context-specific interventions that protect public health and ensure sustainable healthcare.
Activity 2: Understanding the burden of AMR
Before you start this course, think about your own setting. In what ways have you observed the health and economic impacts of AMR? How might these experiences reflect the broader burden of AMR in your country or region? Note down your thoughts in the space below.
Discussion
There are no right or wrong answers to this question; your answers will depend on your role and your setting. You may like to revisit this activity later in the course to see whether your answers have changed.
2 Rationale and value of assessing the burden of disease for AMR
An accurate and precise understanding of the health and economic impact of AMR across countries of all economic levels is important to inform and refine national, regional and global action plans for AMR. Moreover, they are important metrices to inform, monitor and evaluate interventions, including:
- improved access to clean water and sanitation
- infection and prevention and control
- vaccination coverage
- access to and appropriate use of antibiotics.
Finally, the estimate of AMR burden is a key parameter value to inform cost-effectiveness analyses of interventions. This is especially important in helping resource-limited settings to prioritise strategies. The following sections will highlight some examples of utilisation of AMR burden estimates.
2.1 Global and national action plans on AMR
In 2015, the World Health Assembly adopted The Global Action Plan on AMR, which was subsequently endorsed by the Governing Bodies of the Food and Agriculture Organization of the United Nations (FAO), and the World Organisation for Animal Health (WOAH). Together, these bodies and the United Nations Environment Programme (UNEP) formed the Quadripartite.
In 2016, the AMR political declaration adopted by the UN General Assembly agreed on five strategic objectives as the blueprint for addressing AMR globally (UN, 2016). These five strategic objectives are to:
- improve awareness and understanding of AMR through effective communication, education and training
- strengthen the knowledge and evidence base through surveillance and research
- reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures
- optimise the use of antimicrobial medicines in human and animal health
- develop the economic case for sustainable investment that takes account of the needs of all countries and to increase investment in new medicines, diagnostic tools, vaccines and other interventions.
Since then, 178 countries have developed national action plans (NAPs). A new political declaration in September 2024 endorsed by UN member countries included key One Health aims, including:
- a global target to reduce deaths from bacterial AMR by 10% by 2030
- a global target of 70% of antibiotics used for human health to come from the WHO’s ‘Access’ category
- reducing
antimicrobials use in agri-food systems - preventing and addressing the discharge of antimicrobials into the environment.
Burden estimates are a critical component of global and national action plans. They can be used to:
- prioritise actions and resources by helping to identify the most significant threats so that actions and resources can be allocated more efficiently
- inform policy and planning by providing an evidence base for policy decisions
- strengthen surveillance and accountability by supporting the monitoring and evaluation of NAP implementation
- mobilise political and financial support by making a case for investment in tackling AMR
- support global coordination, where standardised burden estimates allow cross-country comparisons which can help to align national efforts with global goals.
Activity 3: AMR burden estimates in your AMR NAP
Go to the World Health Organization’s library of AMR NAPs (WHO, n.d. 4) and find the national action plan for your country.
If a NAP for your country is not available, select one for a country of interest. To get the most out of this activity we suggest that you select a NAP for a country that is in a similar region to your own.
By searching the document, can you identify any AMR burden estimates highlighted in your country’s AMR NAP? You might look for some of the following:
- morbidity estimates such as prevalence, proportion, frequency, incidence rate of AMR
- mortality estimates such as mortality and deaths, and disability-adjusted life years (DALYs)
- healthcare costs
- indirect costs such as loss of productivity or the economic impact on families or communities.
Feel free to copy and paste the relevant sentences into the space below.
Discussion
Most AMR NAPs will include some estimates of morbidity and mortality, but you may also have found some estimates of the direct or indirect economic burden of AMR.
2.2 One Health
AMR is a global threat not only to human health, but also to the health of animals, plants and ecosystems. We live in a connected world and AMR does not need to carry a passport to travel from one area to another. AMR bacteria and antimicrobial resistance genes (ARGs) can spread and transmit within and between humans, animals, plants and the environment.
In 2023, the Quadripartite developed a research agenda to guide One Health AMR research for investment, research activities and planning for countries and funders. The five priority research areas highlighted were:
- transmission
- integrated surveillance
- interventions
- behavioural insights and change
- economics and policy.
The underlying strategic objectives were to:
- improve the understanding of AMR transmission
- strengthen the evidence base for interventions
- collectively use this evidence to advocate for the prioritisation of AMR mitigation and to inform policy.
Burden estimates – including proportion of resistance, frequency of cases, deaths or mortality due to AMR, monetary costs due to AMR, and production loss due to AMR – serve to benchmark and monitor progress towards these objectives.
2.3 Transmission between animal, human and environment
Listed below is a non-exhaustive list of research areas and topics highlighted in the Quadripartite report.The purpose of highlighting these here is to increase awareness of the importance of combating AMR from a One Health perspective. This course will focus on quantifying the AMR burden in human health, but learners should be aware that the transmission of ARGs can happen between animals, humans and the environment, contributing to the AMR burden of each system (see Altevogt et al., 2025).
What is known so far about AMR transmission?
- Several studies have modelled the transmission of AMR in agri-food chains and in human healthcare facilities and clinical settings.
- Microorganisms (including bacteria) can exchange genes, with some restrictions, across interconnected, shared environments of human, animal, plants, water and soils microbiomes.
- Antibiotic resistance genes (ARGs) that are responsible for phenotypic resistance against clinically important antibiotics for disease treatment can cross environmental habitat boundaries and be transferred between non-clinical, environmental microorganisms and human pathogens.
What are the data and research gaps?
- Research on AMR in plant production is still limited.
- There is lack of understanding of transmission dynamics of AMR in low- and middle-income settings.
- The role of the environment as a reservoir of AMR and its impact on human health burden are not fully understood.
- Transmission risk across human, animals, plants, water and soil, and the drivers, still need further exploration.
3 Health outcomes of AMR infection
You have seen the rationale of estimating AMR burden for benchmarking national and global action plans. You will now learn the common terminology used for estimating AMR burden in humans.
3.1 Proportion of AMR infections
Throughout this course, you will see the term ‘
In other words, it is the fraction of patients with a clinical sample culture positive for a resistant phenotype among the total number of patients with at least one positive clinical sample culture for the given organism over a specific period of time.
For example, the proportion of third-generation cephalosporin-resistant (3GCR) Escherichia coli among patients with at least one positive clinical sample culture for E. coli and tested against third-generation cephalosporin (3GC) in 2025 could be calculated using the following equation:
Suppose that among 100 patients with E. coli infections, all their E. coli isolates were tested for susceptibility to 3GCs. Of these, 15 patients had isolates that were resistant to 3GC. The proportion of 3GCR E. coli infections in this cohort can be calculated as:
15/100 = 0.15 or 15%
The term ‘prevalence of AMR’ is also sometimes used in literature to mean ‘proportion of AMR’. ‘Proportion of AMR’ is used in this course to remind you that this measurement of AMR burden is bounded between 0 and 1 (or between 0% and 100% in percentage terms). More importantly, ‘prevalence’ refers to the number of existing disease cases in a given population within a window of time. This is different from ‘incidence’, which refers to the number of new disease cases within a window of time.
One important caveat for proportion of AMR measurements is that a proportion of 30% could reflect three patients with 3GCR E. coli infections in a total number of ten patients with E. coli infections tested for resistant to 3GCs (with high uncertainty and likely a non-representative cohort), or 300 patients with 3GCR E. coli infections in a population of 1000 patients with E. coli infections (with lower uncertainty). Hence it is important that you explicitly report both the numerator (the number of patients with AMR infections) and the denominator (the total number of patients with bacterial infections) when describing the proportion of AMR, or that every time you encounter a reported proportion of AMR, you should ask for the numerator and denominator to support your interpretation.
Moreover, it is important to note that the unit of measurement needs to be consistent for the numerator and denominator. The commonly used units of measure include the number of samples and the number of patients.
A hospital has the following data for the year 2024:
- There were 550 patients who had an E. coli bloodstream infection.
- Of these 550 patients, 450 patients had antimicrobial susceptibility test (AST) results.
- Of these 450 patients who had AST results:
- 150 patients had E. coli isolate(s) identified from at least one clinical sample as resistant to 3GC
- 300 patients had clinical samples with E. coli isolates susceptible to 3GC.
Using the information above, to the nearest whole number what is the estimated proportion of 3GCR E. coli in bloodstream infections at the hospital?
- a.27%
- b.33%
- c.81%
- d.55%
Answer
The correct answer is option (b): 33%. 450 patient samples were tested and of these 150 were found to be resistant to 3GC. The proportion of AMR is calculated as:
3.2 Frequency of AMR infections
While proportions provide a relative measure of prevalence, frequencies describe the absolute rate of infections over a specific time period. Specifying the denominator of the frequency of AMR infections is important for interpretation. Generally, there are two types of denominators:
- population size within the catchment area
- tested population.
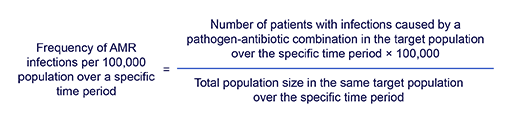
Frequency of AMR infections per 100,000 population per year would be defined as the ratio of the number of patients in the target population with infections caused by a resistant pathogen to the population size within the same target population over a specific period of time. This can be written as follows:
Similarly, frequency of AMR infections per 100,000 tested patients per year would be defined as the ratio of the number of patients in the target population with infections caused by a resistant pathogen to the total number of patients who had at least one clinical sample sent for microbiology testing within the same target population over a specific period of time. This could be written as follows:
For example, in some of the reports or publications you may encounter similar statements such as ‘the frequency of 3GCR Escherichia coli per 100,000 population in 2025 in country X was 5.8’, or ‘the frequency of 3GCR Escherichia coli per 100,000 tested patients in 2025 in Country X was 660’. Both morbidity measurements have the same numerator but different denominators.
Other commonly used terminology includes:
- frequency of AMR infections per 1000 patient days per year
- frequency of AMR infections per 1000 discharge events per year.
Both of these share the same numerator as described above but different denominators.
The term ‘incidence rate of AMR’ is used in the literature and generally refers to the number of new AMR infections occurring in a specific time period. It is conceptually similar to the ‘frequency of AMR’ but specifically focuses on newly identified cases.
3.3 Stratification by origin of infection
When generating data and estimating the proportion and frequency of AMR infections, it is important to stratify the data by origin of infection and to estimate the burden accordingly.
Commonly used categories for origin of infection
There are three categories that are commonly used to stratify data by origin of infection:
- community-origin
- healthcare-associated
- hospital-origin.
The proportion and frequency of AMR infections across these three categories could be very different as patient characteristics and the risk of exposure to AMR pathogens are different.
Community-origin infections have been defined as the isolation of a pathogenic organism from a clinical sample taken within the first two days of hospitalisation and without history of a hospital stay in the 30 days prior to the hospitalisation, or taken from individuals who have signs of infection but do not need hospitalisation (e.g. uncomplicated UTI treated as an outpatient) and without a history of hospital stay in the 30 days prior to the current presentation.
Healthcare-associated infections have been defined as the isolation of a pathogenic organism from a clinical sample taken in the first two days of hospitalisation and with a history of a hospital stay or healthcare interaction within 30 days prior to the hospitalisation.
Hospital-origin infections have been defined as the isolation of a pathogenic organism from a clinical sample taken after the first two days of hospitalisation.
Further details on the classifications and their definition can be found in the article ‘Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals’ (Rodríguez-Baño et al., 2010).
GLASS categories for origin of infection
The World Health Organization (WHO) Global Antimicrobial Resistance and Use Surveillance System (GLASS) (WHO, 2017) defines two types of infection origin as below:
- Community-origin: Patients cared for at outpatient clinics or patients in hospital for two calendar days or fewer when the specimen was taken. Specimens collected from patients in the community or in hospital on the first or second day are considered a proxy for community infections.
- Hospital-origin: Patient admitted for more than two calendar days when the specimen was taken or admitted to the healthcare facility for less than two calendar days but transferred from another healthcare facility where he or she was admitted for two or more calendar days. Specimens collected from hospital patients in hospital on the third day or later are used as a proxy for hospital-origin infections.
Activity 4: Reflecting on measures of AMR in your practice
Thinking about your role and work practice, have you encountered any measures of AMR frequency? Have you noticed any trends in the frequency or occurrence of AMR infections in your workplace?
Discussion
Measures of frequency that you may have come across or calculated might include the total count of the isolates with resistant phenotype in your hospital’s annual AMR report or microbiology database. You might have seen graphs of trend changes (which could suggest an increase, decrease or steady state) over time. It would be useful to discuss the reasons for the observed changes with a colleague.
3.4 The number of deaths and mortality due to AMR
Burden estimates aim to measure the impact of AMR.
A causal interpretation is useful as it tells you how much harm from AMR infections could be prevented if these infections were avoided. This information is crucial for policy-makers to prioritise resources, for the design and evaluation of infection prevention interventions, and to monitor changes in the burden of AMR.
In the literature, you may encounter terminologies including ‘mortality attributable to AMR’, ‘excess mortality due to AMR’ and ‘population attributable mortality’, which could imply a causal interpretation, but a causal interpretation may not always be achievable. There are differences in definition between these terminologies, which you will revisit in the methodology section. While it is important to familiarise yourself with different terminologies used to describe health impact of AMR infections, the key is to understand the analysis approach used and ask yourself if the analysis meant to reach a causal interpretation. It is a good idea to avoid jumping to the conclusion that an estimate holds a causal interpretation just from the terminology used.
3.5 Comparators
When interpreting the health impact of AMR, it is essential to understand the comparator used in the analysis. There are two types of comparators that are commonly used, and these correspond to two distinct definitions of ‘counterfactual scenarios’: the antimicrobial-susceptible infection counterfactual and the AMR infection-free counterfactual. Recall that counterfactual scenarios are hypothetical situations used to understand what would have happened if a specific factor or event (like AMR infections) had not been present.
The antimicrobial-susceptible infection counterfactual
This comparator refers to an equivalent individual with the same infection but caused by the susceptible (rather than resistant) phenotype of the same bacteria. For example, to estimate the number of deaths due to 3GCR infections in a target population over a specified period of time, you would compare to otherwise equivalent individuals with third-generation cephalosporin-susceptible (3GCS) E. coli infection as your comparator.
The underlying assumption when using this comparator is that if resistant infections were prevented, then susceptible infections caused by the same bacterial species could take their place since both compete for the same
The target AMR infection-free counterfactual
This comparator refers to an otherwise equivalent individual who does not have the AMR infection of interest. It includes people with no infections or with infections caused by other pathogens. For example, if you are estimating the number of deaths due to 3GCR infections in a target population over a specified period of time, your comparator group would include all otherwise equivalent individuals without 3GCR E. coli infection, including individuals without an infection and individuals with infections caused by Klebsiella pneumoniae, for example. In other words, this is a counterfactual that is ‘AMR pathogen(s) of interest-free’. Another term used in the literature is the ‘no infection’ counterfactual. It is important to note, however, that this refers specifically to the absence of infection from the AMR pathogen of interest and not of any infection.
The underlying assumption when using this comparator to quantify the impact of AMR infections is that the preventive strategy being considered does not exclusively affect the number or severity of susceptible infections.
There is an ongoing discussion about when to use one comparator over the other and when to use both. The key considerations are the type of intervention one has in mind to reduce the impact of AMR infections, and the underlying mechanism of interaction between AMR pathogens and susceptible pathogens. Details on the empirical evidence and underlying assumption of the two comparators are beyond the scope of this course, but you can follow the WHO attributable mortality protocol (and elsewhere in published literature) to read further. For this course you should just be aware of the two comparators and understand their underlying assumptions.
Your hospital management team is planning to invest in improving water sanitation and hygiene (WASH) by installing new lavatory and clean water pipelines in the hospital wards. A time-series analysis will be performed to monitor the changes in AMR burden. What counterfactual (comparator) would you use to measure AMR burden and why?
Answer
The AMR infection-free counterfactual should be used. An effective WASH intervention is likely to reduce infection rates with many pathogens, regardless of resistance (or antimicrobial susceptibility test) status.
3.6 Years of life lost due to AMR
For example, consider a situation in which ten neonates died due to sepsis caused by 3GCR bacteria in District X, which has a standard life expectancy of 70 years. Each neonate is assumed to have lost 70 years of life due to premature death so for ten neonates the total YLL would be 10 × 70 or 700 life years.
This measurement is an important metric in the calculation of the economic burden of AMR, which will be discussed later in this course.
3.7 Excess length of hospital stays
With a causal interpretation in mind, excess length of hospital stays could be defined as the difference between the observed length of hospital stay and the expected length of hospital stay that would be observed in the absence of an AMR exposure of interest.
This measurement is an important metric in the calculation of the economic burden of AMR, which will be discussed later in this course.
Now that you have reviewed the common terminology used for estimating AMR burden in humans, try Activity 5 to check your understanding.
Activity 5: Assessing your understanding of terminology
4 Data sources and methodologies for measuring burden of AMR
In the previous section you saw some of the commonly used epidemiological terminologies and their definitions to describe health outcomes of AMR. In this section you will look at some existing data sources and analytical methods used to estimate burden of AMR. You will also learn how to interpret the estimates and review some of important caveats and limitations.
4.1 Estimating the proportion and frequency of AMR
The following list reviews the data sources available for estimating the proportion and frequency of AMR, including information on the analytical approaches used for each source, as well as their strengths and limitations:
- Isolate-based surveillance data, such as routinely collected microbiology results, is often used to calculate the proportion of AMR infections. The data is often available in electronic format, making it easy to access and analyse with minimal effort. However, it usually relies on blood samples, which may not capture the full range of infections like respiratory or urinary tract infections – especially in settings where clinical data is limited or not well structured. Additionally, low use of microbiology testing services can introduce bias, and hospital data is often needed to estimate infection frequency and to break down results by infection origin, age or sex.
- Prospective case-based surveillance data involves actively collecting data on patients who meet specific infection definitions. It allows for direct calculation of both the proportion and frequency of AMR infections and provides a detailed understanding of different infection types. However, it is resource-intensive, requiring significant investment in staff and infrastructure to identify and enrol patients and collect data.
- Point-prevalence surveillance survey data is collected at a specific point in time and offers a snapshot of AMR infection rates at a specific time, using moderate resources. It can complement routine data and help estimate both proportion and frequency of infections. However, results may not represent the full year, and sample sizes can be small. These surveys often underrepresent community-origin infections and short-stay hospital patients, leading to potential sampling bias.
- Systematic reviews use meta-analysis, a statistical method that combines and analyses the results of multiple studies to summarise AMR infection data from multiple studies. They are cost-effective, since they rely on existing data. However, differences in study quality, settings and methods can introduce bias. Additionally, most studies come from tertiary hospitals, which may not reflect national or regional patterns.
- Combining multiple data sources using advanced statistical models (e.g. machine learning or decision trees) can help estimate AMR burden in settings with limited data. This approach benefits from large data volumes, but faces challenges like data inconsistency, varying quality and assumptions about similarities between countries that may not be accurate. Community-origin infections and non-tertiary hospitals are often underrepresented.
4.2 Interpreting estimates
When you read and review reports or publications on the proportion and frequency of AMR infections, it is important to make sure that you understand how the numerators and denominators are estimated. When interpreting the estimates you should ask yourself the following key questions:
- Are the estimates biased towards patients with specific characteristics, such as patients who failed initial empiric antibiotic treatments?
- Are the denominators appropriately selected?
- Do the estimates represent the target population?
If you are collecting data and/or calculating and reporting proportion and frequency of AMR infections, it is useful to be aware of the tools and protocols available to support you when you are processing data and generating reports. Some examples that you might like to explore include the following:
- WHONET, an application for management and analysis of microbiology laboratory data.
- SEDRI- LIMS (Global Laboratory eTools, n.d.), an open source laboratory information management system specialising in microbiology.
- AMASS, an open access offline application to automatically generate AMR surveillance reports using microbiology data and, if available, hospital admission data.
- MICRO (Turner et al., 2019), a checklist to guide the reporting and interpretation of clinical microbiology data.
- AMR (for R), a package within R software for AMR data analysis.
It is also useful to be aware of data-generation and reporting systems in local, national and regional AMR networks and protocols.
Whenever possible you should consider reporting:
- the numerators and denominators of your estimates
- the number and proportion of missing antimicrobial susceptibility test results for the antibiotics under analysis among the cases with the pathogen of interest identified
- stratified data or estimates by community-origin, healthcare-associated and hospital-origin, because each of these has distinct population characteristics, transmission dynamics and burden of AMR, hence would require different strategies to control spread of AMR
- stratified data or estimates by sex and age groups – you can find out more about the importance of disaggregated data in the Gender and equity in AMR surveillance course
- metadata that could reflect the data sources (i.e. number and type of healthcare facilities that serve the target population from which data was retrieved), catchment population size, microbiology testing service utilisation rate (i.e. number of culture sets sent for microbiology testing over the reporting period), total patient days over the reporting period, total number of discharges over the reporting period, etc.
4.3 Estimating mortality and number of deaths due to AMR
Table 1 contains information on the data sources available for estimating the mortality and number of deaths due to AMR as well as their strengths and limitations.
Note on choosing comparators for community-origin AMR infections
An important (and often neglected) consideration when estimating deaths due to community-origin AMR infections is the choice of data source from which the comparator cohort comes and represents.
For example, if the aim is to calculate excess deaths due to community-origin AMR compared with the AMR infection-free counterfactual (see definition in the previous section), it is important to note that patients admitted to hospital without community-origin infections will not be an appropriate comparator. This is because patients admitted to hospital for other conditions (such as stroke, surgery, accidents, etc.) may be expected to have a higher mortality risk than the general population in this community. Instead, you would need a cohort of individuals from the same community as those with community-origin AMR infections but without bacterial infections.
| Measurements | Data sources | Gaps and limitations |
|---|---|---|
| Crude mortality | Death registry data Hospital clinical data International Statistical Classification of Disease (ICD) code data | ICD data may underestimate the number of deaths related to AMR. This is because mortality statistics often allow selection of only one main underlying cause of death, which means that only the underlying condition that led to the hospital admission would be featured as cause of death, instead of other intercurrent conditions such as infections (e.g. hospital-acquired bacterial infections). |
| Mortality due to AMR infection using clinical definitions | Medical charts | Time-consuming; also, clinical definition of mortality due to AMR can be subjective. |
Estimation of excess mortality due to AMR Population attributable mortality due to AMR | A comprehensive dataset that contains individual-level clinical, microbiology and outcome data for modelling There are different study designs used to generate data, ranging from summaries of published analyses through systematic reviews to case-control studies, cohort studies and vaccine-probe trials* | Can be computationally challenging to perform analysis locally. To derive an unbiased estimate of this measurement, good quality, high-dimensional, detailed and large sample size data would often be needed; that is, representative, complete and accurate microbiology test result data, clinical data that captures pre-defined confounders, and patient treatment outcome data. |
Footnotes
*The design and analytical approach of vaccine-probe trials are beyond the scope of this course, but if you’re interested in this area you might want to refer to Feikin et al.’s review article ‘Use of vaccines as probes to define disease burden’ (2014). Examples of use of this design include for the burden of pneumococcal pneumonia disease, which remains an important bacterial infection for children in many low- and middle-income countries.Table 2 builds on the data sources and methods outlined in Table 1 and covers the corresponding analytical approaches used to interpret each measurement and their implications for estimating the health impact of AMR.
| Measurements | Analytical approaches | Interpretations |
|---|---|---|
| Crude mortality | Numerator: total counts of deaths with AMR infection in a target population over a specific time period. Denominator: total number of patients with AMR infections in the same target population over the same time period. | In some literature this is used interchangeably with the case fatality ratio. There is no causal interpretation to this measurement. Often it is challenging to compare this measurement between different settings or between different time points in the same setting; this is because difference could be due to many factors, including patient characteristics and heterogeneity in a third variable that could influence the risk of AMR infection and mortality. |
| Mortality due to AMR infection using clinical definitions | Numerator: the number of deaths that are clinically defined as directly or indirectly due to AMR infection in a target population over a specific time period. Denominator: total number of patients clinically suspected of bacterial infection and confirmed by microbiology culture that AMR pathogen(s) of interest was/were present in at least one clinical sample in the same target population over the same time period. | This is not a common measure of health impact due to AMR infections for reasons of practicality. This measurement could have an individual-level causal interpretation.* However, the clinical definition of death due to AMR infection can be subjective, which impedes the ability to compare across settings or across time. |
| Estimation of excess mortality due to AMR | Often estimated using modelling approaches | An unbiased** estimate of this measurement has a population-level causal interpretation to inform the preventable risk of mortality that would not have occurred had individuals not experienced AMR infection. This is the absolute difference between the observed risk of mortality among individuals who were factually exposed (i.e. had an AMR infection) and the probability of mortality that there would have been in the absence of AMR infection. |
| Population attributable mortality due to AMR | Often estimated using modelling approaches | An unbiased** estimate of this measurement has a population-level causal interpretation that informs the proportion of deaths that would not have occurred in the absence of AMR infection in the total population. This is often used a key parameter to estimate the number of deaths due to AMR infection. |
| Number of deaths due to AMR infection | This could be calculated by multiplying the estimated number of cases of AMR infection by excess mortality due to AMR. | An unbiased** estimate of this measurement has a population-level causal interpretation to inform the preventable number of deaths that would not have occurred had individuals not experienced AMR infection. |
Footnotes
*Please refer to the section on common terminologies for health burden of AMR in this course for detailed definition and underlying assumption of the two comparators. **It is often challenging to derive an unbiased estimate in practice and interpretation needs to carefully consider different source of biases.4.4 Interpretation based on choice of comparator
The interpretation of deaths due to AMR (whether referring to excess mortality, population attributable mortality or deaths due to AMR) has two components based on the comparator used. (For the definitions of the two comparators and their underlying assumptions refer to Section 3.5.)
Using the antimicrobial-susceptible infection counterfactual, this estimate reflects the number of preventable deaths assuming that:
- AMR infections could be avoided
- susceptible infections of the same bacterial species could take over the same ecological niche.
Using the target AMR infection-free counterfactual, this estimate reflects the number of preventable deaths assuming that:
- AMR infections could be avoided
- preventing the AMR infection would not increase the risk of susceptible infections caused by the same pathogen.
Activity 6: Contextualising the scale of AMR burden and reflecting on burden of AMR for your country
The following two tasks aim to help you contextualise AMR burden estimates. You will be using two open access platforms to take a close look at the existing estimates for your country.
The first platform is the WHO’s Global Antimicrobial Resistance and Use Surveillance System (GLASS) surveillance dashboard. Launched in 2015, WHO GLASS is the first global collaborative effort to standardise AMR surveillance. The WHO has provided further details of WHO GLASS (WHO, n.d. 3).
The second platform is the Measuring Infectious Causes and Resistance Outcomes for Burden Estimation (MICROBE) interactive visualisation tool. This tool is one of the outputs from the Global Research on Antimicrobial Resistance (GRAM) project, which aims to understand the threat using modelling to estimate the burden of AMR. The University of Washington’s Institute for Health Metrics and Evaluation has provided further details of the GRAM project (IHME, n.d.). A key publication from the GRAM project is ‘Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis’ (AMRC, 2022).
Task 2
Go to the MICROBE tool.
Select your country from the drop-down menu and complete Table 3 using the information for your country. Explore the dashboard by reading the descriptions for each of the presented estimates and by going through the ‘Sepsis’, ‘Bacteria’ and ‘Resistance’ tabs.
| Country | |
| Estimated number of sepsis deaths | |
| Number of sepsis deaths related to bacterial infections | |
| Number of excess deaths due to AMR infection compared to if the exposed population had an antimicrobial-susceptible infection | |
| Number of excess deaths due to AMR infection compared to if the exposed population had not had any bacterial infection |
(Optional) Fill in the table for another country and reflect on why there might be differences between this country and your own.
Discussion
The results that you recorded in Table 3 will depend on the country that you have selected. Table 4 shows the data for Timor-Leste as an example.
| Country | Timor-Leste |
| Estimated number of sepsis deaths | It is estimated that in 2021, 3423 people in Timor-Leste died with sepsis as an immediate or intermediate cause of death |
| Number of sepsis deaths related to bacterial infections | The number of bacterial infections in the sepsis deaths is 1851 |
| Number of excess deaths due to AMR infection compared to if the exposed population had an antimicrobial-susceptible infection | 192 deaths |
| Number of excess deaths due to AMR infection compared to if the exposed population had not had any bacterial infection | 840 deaths |
Now that you have learned about how the burden of AMR can be estimated, the next sections of the course will show how these techniques can be (a) linked to economics, and (b) used to inform decision-making.
5 The economic impact of AMR
The implications of AMR are substantial for global and national economies for the following reasons:
- AMR infections in the healthcare sector are typically more expensive to treat than non-resistant (or susceptible) infections. This is because of one or a combination of extended hospital stays, more expensive pharmaceutical treatments and additional diagnostic processes. Inappropriate treatment can also lead to recurrence of infections, leading to further costs.
- AMR in the agriculture sector leads to higher livestock production costs because antibiotics are often used as prophylaxis, meaning that more expensive antibiotics are required. Resistant outbreaks can lead to increased culling and are a threat to food security and the livelihoods of producers (Martins et al., 2024).
- The economic impact of AMR is also felt beyond the healthcare sector. Societal economic losses through reduced productivity and increased absenteeism occur as a result of prolonged or recurrent infections. Furthermore, increased health costs can increase the likelihood of financial hardship or catastrophic health expenditure for sick individuals if they have to pay for medical care themselves. This can cause longer-term impoverishment, particularly when they are forced to sell any assets (such as housing or livestock) to cover medical costs.
There has been a lot of research conducted on the economic burden of AMR if left unaddressed. One of the most influential pieces of work in this area is the O’Neill Report (Review on Antimicrobial Resistance, 2016), which included a projection that by 2050, 10 million deaths a year would occur as a result of AMR, with the global economy losing $100 trillion by the same year.
Although these figures were later disputed (de Kraker, 2016), there have since been many other projections of the economic burden of AMR, with a consistent message: if AMR is not curtailed, its impact will be enormous. The World Bank estimated that by 2050, unaddressed AMR could reduce global GDP by 3.8% each year and increase the number of people in poverty by 28 million globally (World Bank Group, 2024). A recent systematic review by Poudel et al. (2023) of the burden of resistant infections compared to susceptible infections found that the mean incremental cost per patient episode ranged from –$2,371 to $29,289 (in 2020 USD); only 1 of the 29 studies reported statistically significant lower costs for resistant infections.
Meanwhile, the extension of patient length of stay due to resistant infections was 7.4 days. It is worth noting that most of the studies identified in this review were from high-income settings, though there were some studies in lower-income countries identified.
The additional costs of resistance per patient are often greater in absolute terms in high-income settings (Poudel et al., 2023), likely due to greater costs of resources and labour; however, in relative terms this may not be the case. Despite this, low- and middle-income countries are likely to be more impacted by rising AMR for the following reasons:
- Interventions to combat resistant infections require additional resources, such as antimicrobial stewardship programs, infection prevention control measures, or surveillance programs. Resources in these settings are typically more constrained than in high-income settings.
- AMR routinely raises the costs of delivering healthcare services, for example due to extended hospital stays, additional diagnostic and microbiology utilisation, or higher costing alternative antibiotics to treat resistant infections. The healthcare system may not have the capacity or infrastructure to manage these additional demands, and more expensive antibiotics may not be affordable or even available in lower-resource settings.
- The health economic impact of AMR is also exacerbated by issues of access to vaccines, diagnostics and treatments, among other issues.
5.1 An introduction to health economics
A key part of economics is the study of decision-making under conditions of scarce resources.
Financial budgets are invariably constrained, so this is often the focus in health economics. However, other resources – such as time, labour and equipment – can also be considered. Health economists often consider both the health and cost consequences of decisions.
There are many different types of health economic analyses, but you will only focus on two types in this course (Turner et al., 2021):
- Costs of illness or burden of disease estimates aim to quantify or estimate the financial or health impacts of a disease over a defined period of time. These estimates can also be used to estimate the costs or burden in the future, based on estimates of future incidence – the economic estimates in the O’Neill report discussed earlier in the course would fit in this category. Importantly, these analyses do not estimate the impact of any interventions, and the extent to which these costs or burdens may be reduced.
- Cost-effectiveness analyses are a form of comparative economic analysis that consider two or more policies – a new intervention compared to current practice, for example – and estimate the costs and outcomes associated with each approach. As such, these analyses can inform which approaches represent the best value for money and can guide the efficient use of healthcare budgets.
5.2 Costs
Economic costs in health economics refers to the monetary value of resources utilised as well as the
In health economics there are two types of costs to consider: direct and indirect (Turner et al., 2023).
- Direct costs, specifically direct medical costs, are the explicit costs that go towards medical care, such as healthcare staff time, equipment, medication and consumables. Direct non-medical costs are the additional costs associated with medical care that are incurred as a result of illness separate to medical treatment costs but are related to receiving healthcare. Examples include costs related to transportation, accommodation and meals.
Indirect costs include the costs that are incurred as a result of illness but are not directly a result of seeking healthcare. These include medical and non-medical costs, although indirect medical costs are not often considered. Indirect non-medical costs are more frequently included in economic analyses such as productivity losses: for example, from death, the patient’s inability to work whilst sick or a caregiver’s inability to work in order to care for an ill person.
In the case of AMR, the use of antibiotics is accepted as a driver of future AMR, so there is an extra societal cost associated with the consumption of antibiotics today with the incidence of future resistance and the associated costs of future resistant infections. This is a very complicated cost to estimate, although some attempts have been made by researchers (Shrestha et al., 2018).
Video 1 explains the difference between direct and indirect costs.
5.3 Quantifying health outcomes in economics
A key issue in health economics is how to most productively deploy limited healthcare resources. These decisions necessarily involve weighing the health impacts of distinct interventions against each other. For example, decision-makers have to decide whether intervention to reduce AMR is more important to implement than an intervention for tuberculosis or the hepatitis C virus.
As such, universal measures were developed in an attempt to quantify impacts on all health conditions in a fair and consistent way. To do so, these measures need to consider that health burdens can be realised in two ways:
- mortality (i.e. health conditions that can cause premature death)
- morbidity (i.e. health conditions that can have symptoms or contribute to poor health whilst alive).
The two most commonly used measures of this type are disability-adjusted life years (DALYs) and quality-adjusted life years (QALYs). DALYs are more commonly used in low- and middle-income contexts and, as such, are what you are focusing on in this course, but more information about QALYs can be found elsewhere (De Silva et al., 2023). Alternatively, some economic analyses use other natural units to quantify the health outcomes, such as number of deaths, number of new disease cases or number of antibiotics prescribed.
There have also been recent efforts to develop additional outcome metrics that reflect the One Health nature of AMR. For example, researchers developed a modified DALY metric for zoonotic diseases, known as the zDALY, that considers both human health and the impact of poor animal health on its owner in terms of the time that might be required to replace that animal. Further information on the zDALY can be found online (Torgerson et al., 2018).
5.4 Disability-adjusted life years (DALYs)
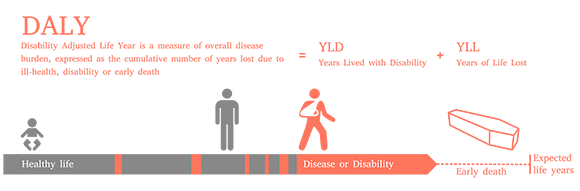
DALYs are a quantitative measure of burden of disease that reflects the total amount of good health lost, whether from premature mortality or poor health and disability over a period of time.
DALYs for a health condition can be calculated by adding the healthy years of life lost due to poor health or disability to the years of life lost due to premature mortality. This calculation is DALY = YLD + YLL, where YLD = years lived with disability and YLL = years of life lost.
For the proportion of patients that die, the YLLs lost can typically be calculated by subtracting the life expectancy with a health condition from the normal life expectancy in that country or setting. The YLDs are calculated by multiplying the time spent living with disability by a factor that accounts for the severity of the condition with something called a ‘disability weight’.
5.5 Quantifying severity of disability
As previously mentioned, the YLDs in DALYs are calculated using a numerical factor or weight that accounts for the severity of the health condition. These disability weights are measured on a scale from 0 to 1, where 0 equals a state of full health and 1 is equivalent to death.
Helpfully, the Global Burden of Disease study produced a set of disability weights that can be used for economic evaluations (GBD, 2021). The disability weights in this dataset cover a broad range of health conditions and can be applied to any country or setting.
Activity 7: Calculating DALYs from a sample dataset
Look at the information in Table 5, which presents information on four healthcare strategies for a health condition. The strategies include current practice and three new strategies (A, B and C). The strategies are all used for the same type of patients and impact both their life expectancy and the symptoms of the disease.
Calculate the estimated number of DALYs for each of the four strategies by completing the ‘DALYs’ column in Table 5.
Additional key information: The average life expectancy for the general population is 65 years, and the disability weighting for this health condition is 0.4.
Hint: Remember the information from Section 5.4 about how to calculate DALYs. As a reminder, the formula is DALY = YLD + YLL, where YLD = years lived with disability and YLL = years of life lost.
| Interventions | Number of years lived (age at death) | Years of life lived with symptoms | DALYs |
|---|---|---|---|
| Current practice | 55 | 10 | |
| Strategy A | 55 | 6 | |
| Strategy B | 60 | 7 | |
| Strategy C | 58 | 5 |
Discussion
Table 6 shows the completed table.
| Interventions | Number of years lived (age at death) | Years of life lived with symptoms | DALYs |
|---|---|---|---|
| Current practice | 55 | 10 | 14 |
| Strategy A | 55 | 6 | 12.4 |
| Strategy B | 60 | 7 | 7.8 |
| Strategy C | 58 | 5 | 9 |
To calculate the DALY, first the YLD and YLL must be calculated. YLD is calculated by multiplying the number of years of life lived with symptoms by the disability weighting. For example, using the information in the question and in Table 5:
YLD = 10 × 0.4 = 4
YLL is calculated by subtracting the life expectancy with a health condition from the normal life expectancy. For example, using the information in the question and in Table 5:
YLL = 65 – 55 = 10
Now the DALY can be calculated as:
DALY = YLD + YLL
This would be:
DALY = 4 + 10 = 14
5.6 Cost–benefit analyses
Cost–benefit analyses (CBAs) use similar principles to cost-effectiveness analyses, and are a common approach used to analyse the effect of policies outside of the healthcare sector. The key differences between CBAs and cost-effectiveness analyses is that all health outcomes are expressed in monetary values.
In certain contexts, policy-makers may be more familiar or accepting of economic evidence that takes the form of a CBA. One key advantage of a CBA is that it allows a comparison of value for money between health and non-health interventions. In other words, the value for money of a healthcare policy can be compared to a change in policy in the environmental or education sector – this would be useful, for example, for a Ministry of Finance.
However, in practice, health-focused CBAs often omit broader societal costs or rely on contested monetary valuations of health outcomes, which can limit their comparability and relevance. Because AMR strategies should take a One Health approach and involve multi-sectoral stakeholders, there may be circumstances where CBAs are a more appropriate approach to inform decision-making than cost-effectiveness analyses: for example, such as when the decision-makers or funders have a mandate beyond human health.
6 Determining cost-effectiveness
Now that you have been introduced to the concept of health economic analysis, the following sections will explore how cost-effectiveness is determined and how different strategies or interventions can be compared to determine which is the most cost-effective.
6.1 Incremental cost-effectiveness ratios
A key metric in cost-effectiveness analyses is the
Health outcomes can be expressed in any form defined by the economic analysis, such as DALYs, QALYs, deaths or cases. Incremental costs are the costs of one intervention minus the costs of the comparator strategy.
Similarly, incremental health outcomes are the health outcomes of one intervention minus the health outcomes of the comparator strategy. ICERs are then reported as the additional cost per health unit that is gained; for example, cost per DALY averted, or cost per case averted. The ICER calculation can be rearranged to calculate the net monetary benefit (NMB), another key metric that is not explored further in this course.
6.2 Cost-effectiveness thresholds
ICERs provide an estimate of cost-effectiveness but cannot alone necessarily be used to understand whether an intervention is cost-effective.
An ICER could be compared to a
In principle the CET is meant to represent the opportunity cost of health spending in a country. Some countries have explicit CETs that have been endorsed by decision-makers, such as the UK and Thailand (Bertram et al., 2016). There are other countries that have implicit CETs, but they do not reveal the precise value. Many countries do not have defined CETs; however, there are researcher-generated estimates of CETs for most countries that can be used to understand whether a setting may be cost-effective in these settings (Woods et al. 2016; Ochalek, Lomas and Claxton, 2018). Using a value of 1–3 × gross domestic profit (GDP) per capita used to be a recommended threshold by the WHO and although it is no longer recommended, this threshold is still commonly used (Kazibwe et al., 2022).
6.3 Decision-making using the cost-effectiveness plane
The
- the difference in health outcomes (e.g. DALYs) on the x-axis
- the difference in costs on the y-axis.
Each point on the plane represents the comparison between two interventions.
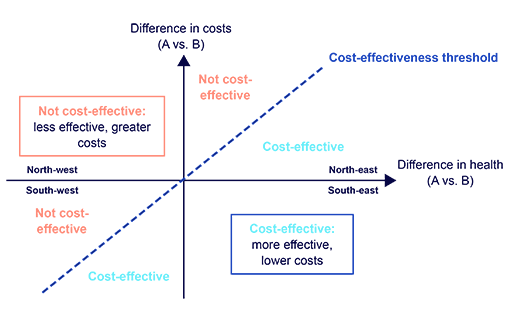
Figure 6 shows an example of a cost-effectiveness plane for two hypothetical interventions, A and B. The plane is divided into four quadrants, each with a distinct interpretation:
- North-west (top left): Intervention A is more expensive and yields fewer health outcomes than Intervention B. Intervention A is therefore not cost-effective and does not need to be compared to a cost-effectiveness threshold. In health economics terminology, Intervention A is ‘dominated’ by Intervention B.
- North-east (top right): Intervention A is more expensive and yields more health outcomes than Intervention B. If the ICER of Intervention A versus Intervention B falls below the CET value, then Intervention A is cost-effective; if not, it is not cost-effective.
- South-east (bottom right): Intervention A is less expensive and yields more health outcomes than Intervention B. Intervention A is therefore cost-effective and does not need to be compared to a cost-effectiveness threshold. In health economics terminology, Intervention A is ‘dominant’ of Intervention B.
- South-west (bottom left): Intervention A is less expensive and yields fewer health outcomes than Intervention B. In this uncommon circumstance, the interpretation of the ICER versus CET is reversed: if the ICER of Intervention A versus Intervention B is above the CET value, then Intervention A is cost-effective; if not, it is not cost-effective.
6.4 Using economic evidence to prioritise interventions
Now that you have seen how incremental cost-effectiveness ratios can be used to compare interventions, try Activity 8 to put what you have learned into practice.
Activity 8: Calculating incremental cost-effectiveness ratios
Table 7 includes information about four healthcare strategies for a hospital to reduce the incidence of hospital-acquired infections. The strategies include current practice and three new strategies (a hand hygiene programme and two screening programs, A and B).
Using the information in Table 7, try to complete the second column in Table 8 by calculating the incremental cost-effectiveness ratios (ICERs) using the equation below. (You should round your answers to the nearest dollar.)
Note that the order of the ICER formula is different in the numerator and denominator when DALYs are used, as greater DALYs indicates worse health outcomes. Therefore, the formula is adjusted to provide cost per DALY reduction. The ICER for cost per DALY averted can be calculated using the following equation:
Having calculated the ICER for each of the pairs of strategies in Table 7, compare the ICERs to the cost-effectiveness threshold to understand whether the strategies are effective compared to the comparator. You need to remember the cost-effectiveness plane here. Record your decisions in the final column of Table 7.
The cost-effectiveness threshold is $9300/DALY averted.
| Interventions | Total cost ($) | Total DALYs |
|---|---|---|
| Current practice | 0 | 15 |
| Hand hygiene programme | 10,000 | 13 |
| Screening programme A | 50,000 | 10 |
| Screening programme B | 60,000 | 7.5 |
| Cost-effectiveness | ICER ($/DALY averted) | Cost-effective? (Yes/No) |
|---|---|---|
| Hand hygiene programme versus current practice | ||
| Screening programme A. versus current practice | ||
| Screening programme B versus current practice | ||
| Screening programme B versus hand hygiene programme |
Discussion
Table 9 shows the correctly completed table.
| Cost-effectiveness | ICER ($/DALY averted) | Cost-effective? (Yes/No) |
|---|---|---|
| Hand hygiene programme versus current practice | 5000 | Yes |
| Screening programme A. versus current practice | 10,000 | No |
| Screening programme B versus current practice | 8000 | Yes |
| Screening programme B versus hand hygiene programme | 9091 | Yes |
Here’s an example ICER calculation for hand hygiene programme (HH) versus current practice (CP):
Screening programme A was found not to be cost-effective compared to current practice and thus was not compared to the two cost-effective interventions (the hand hygiene programme and screening programme B).
Use your completed Table 6 to answer the following questions:
- Solely using the cost-effectiveness evidence, if only one strategy could be implemented, which should it be?
- Hand hygiene programme
- Screening programme A
- Screening programme B
- Imagine that you have now learned that there are concerns that some costs for screening programme B were omitted from the cost-effectiveness analysis. How might this change your answer?
Discussion
There is no correct answer to the first question, but you might have considered that the ICER of screening programme B versus the hand hygiene programme is less than, but very close to, the CET. Therefore, any increase in the costs of screening programme B will bring the ICER closer to the CET or potentially exceed it, meaning that there is a chance that screening programme B would no longer be estimated to be cost-effective compared to the hand hygiene programme.
7 Costs and benefits of strategies to mitigate AMR
To better understand how cost-effectiveness is applied to efforts to mitigate AMR, you are now going to look at a published cost-effectiveness analysis. There are three analyses to choose from, which you can access using the links below. Each considers a different type of intervention relevant to AMR:
- A hand hygiene programme in Mexico (Salinas-Escudero et al., 2023).
- Using whole genome sequencing in Australia (Elliott et al., 2021).
- An antimicrobial stewardship programme in Ethiopia (Gebretekle et al., 2021).
You do not need to read all these case studies; you should select the one that interests you most to complete Activity 9. You can answer the first questions by reading only the abstracts; if you want to spend more time on this activity, you can answer additional questions by reading the sections in the articles on methods, discussions and conclusions.
Activity 9: Evaluating costs and benefits of strategies to mitigate AMR in case studies
Once you’ve chosen the article that interests you most, answer the following questions in the space below:
- Which strategies or interventions were compared in the analysis?
- Did the analysis quantify health outcomes in terms of QALYs or DALYs? If not, what type of health outcome was used instead?
- Did the authors conclude whether the interventions were cost-effective?
You may also want to consider the following optional further questions:
- What kind of costs were included in the analysis? Do you think any were not recognised?
- Did the researchers use a cost-effectiveness threshold? If so, what was it?
- What type of data for the effectiveness of interventions were used, and do you think this was appropriate?
- What were the key strengths and limitations of the research?
- What was the conclusion of the paper? Do you think the conclusions were justified and generalisable to other contexts (such as your own country)?
Discussion
If you focused on the article about the hand hygiene programme in Mexico (Salinas-Escudero et al., 2023), you may have the following answers:
- The analysis compared the implementation of an automated hand-hygiene monitoring system versus non-implementation.
- It did not use DALYs or QALYs; health outcomes were quantified in terms of the number of infections.
- The automated hand-hygiene monitoring system was estimated to be cost-saving and more effective (with reduced infections), and therefore ‘dominant’.
If you focused on the article about using whole genome sequencing in Australia (Elliott et al. 2021), you may have the following answers:
- The analysis compared three scenarios: use of whole genome sequencing and metagenomics; use of just whole genome sequencing; and no whole genome sequencing or metagenomics.
- It used QALYs to quantify health outcomes.
- It estimated that both intervention scenarios were likely to be cost-effective, with whole genome sequencing and metagenomics estimated to be the most cost-effective strategy.
If you focused on the article about the antimicrobial stewardship programme in Ethiopia (Gebretekle et al., 2021), you may have the following answers:
- The analysis compared pharmacist-led antimicrobial stewardship (with concurrent strengthening of laboratory capacity) with usual care.
- It used QALYs and expected life years to quantify health outcomes.
- The antimicrobial stewardship programme was estimated to be cost-saving and more effective (yielding more QALYs), and therefore cost-effective and dominant.
8 Communicating the health and economic burden of AMR in your work
You should now have a better understanding of how the health and economic burden of AMR is conceptualised and can be measured or estimated. Before you finish this course, you should complete this final activity, which will help you to think about your role in communicating these concepts and analyses to your peers and stakeholders.
Activity 10: Health and economic burden of AMR in your work
Think about what you have learned in this course. Use the space below to reflect and make notes in response to the following questions:
- How might you and/or your colleagues apply your knowledge of the health and economic burden of AMR to your workplace?
- How would you now approach antimicrobial decision-making differently, now that you understand the broader health and economic consequences of resistance?
- Would you feel more comfortable communicating estimates of the health and economic burden of AMR through your work and to your colleagues?
9 End-of-course quiz
Well done – you have reached the end of this course and can now do the quiz to test your learning.
This quiz is an opportunity for you to reflect on what you have learned rather than a test, and you can revisit it as many times as you like.
Open the quiz in a new tab or window by holding down ‘Ctrl’ (or ‘Cmd’ on a Mac) when you click on the link.
10 Summary
In this course you have learned the basics of estimating the health and economic burden of AMR. You have learned about measuring the impact of bacterial AMR on populations, geographies and individuals. You should now have an understanding of the significant global impact that AMR has on public health and economies, and how policies to address AMR can be evaluated from a health economic perspective.
You should now be able to:
- define key epidemiological terminologies and concepts related to the burden of bacterial AMR
- explain the rationale and value of assessing the burden of disease for AMR
- demonstrate an understanding of the metrics and indicators that are commonly used to describe the burden of AMR
- outline how data collected and analysed using available methodologies for estimating the burden of AMR can be interpreted, highlighting the strengths and limitations of each methodology
- reflect on burden of disease data related to AMR in your settings (including community, hospital, country, and WHO region) and at a global level
- demonstrate an awareness of the direct and indirect economic consequences of AMR and antimicrobial use on healthcare systems, individuals, communities and economies, considering both short- and long-term perspectives
- outline the benefits and costs of One Health strategies to mitigate the effects of AMR
- explain how economic analysis can inform AMR-related policy decisions by identifying interventions that provide the greatest benefit relative to their costs.
Now that you have completed this course, consider the following questions:
- What is the single most important lesson that you have taken away from this course?
- How relevant is it to your work?
- Can you suggest ways in which this new knowledge can benefit your practice?
When you have reflected on these, go to your reflective blog and note down your thoughts.
Activity 11: Reflecting on your progress
Do you remember at the beginning of this course you were asked to take a moment to think about these learning outcomes and how confident you felt about your knowledge and skills in these areas?
Now that you have completed this course, take some time to reflect on your progress and use the interactive tool to rate your confidence in these areas using the following scale:
- 5 Very confident
- 4 Confident
- 3 Neither confident nor not confident
- 2 Not very confident
- 1 Not at all confident
Try to use the full range of ratings shown above to rate yourself:
When you have reflected on your answers and your progress on this course, go to your reflective blog and note down your thoughts.
11 Your experience of this course
You’ve now reached the end of this course. If you’ve enrolled on a pathway, please go back to the pathway page and tick the box to confirm that you’ve completed this course. On the pathway page you’ll see both your progress so far as well as the other courses you need to complete in order to achieve your Certificate of Completion for that pathway.
Now that you have completed this course, take a few moments to reflect on your experience of working through it. Please complete a survey to tell us about your reflections. Your responses will allow us to gauge how useful you have found this course and how effectively you have engaged with the content. We will also use your feedback on this pathway to better inform the design of future online experiences for our learners.
Many thanks for your help.
References
Aiken, A.M., Rehman, A.M., de Kraker, M.E.A., Madrid, L., Kebede, M., Labi, A.-K., Obeng-Nkrumah, N., Nyamwaya, B., Kagucia, E., Cocker, D., Kawaza, K., Lester, R., Iregbu, K.C., Medugu, N., Nwajiobi-Princewill, P.I., Dramowski, A., Sonda, T., Hemed, A., Fwoloshi, S., Ojok, D., Scott, J.A.G. and Whitelaw, A. (2023) ‘Mortality associated with third-generation cephalosporin resistance in Enterobacterales bloodstream infections at eight sub-Saharan African hospitals (MBIRA): a prospective cohort study’, The Lancet: Infectious Diseases, 23(11), pp. 1280–90, November [online]. Available at https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00233-5/fulltext (accessed 9 Septeember 2025).
Altevogt, B.M., Taylor, P., Akwar, H.T., Graham, D.W., Ogilvie, L.A., Duffy, E. and Essack, S.Y. (2025) ‘A One Health framework for global and local stewardship across the antimicrobial lifecycle’, Communications Medicine, 5, article number 414, 7 October [online]. Available at https://www.nature.com/articles/s43856-025-01090-4 (accessed 17 October 2025).
AMASS, https://amass.website/infobox.aspx?pageID=101 (accessed 9 September 2025).
AMR (for R), https://amr-for-r.org/ (accessed 9 September 2025).
Antimicrobial Resistance Collaborators (AMRC) (2022) ‘Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis’, The Lancet, 399(10325), pp. 629–55, 12 February [online]. Available at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext (accessed 9 September 2025).
Bertram, M.Y., Lauer, J.A., De Joncheere, K., Edejer, T., Hutubessy, R., Kienya, M.-P. and Hilla, S.R. (2016) ‘Cost–effectiveness thresholds: pros and cons’, Bulletin of the World Health Organization, 94(12), pp. 925–30 [online]. Available at https://doi.org/10.2471/blt.15.164418 (accessed 10 September 2025).
de Kraker, M.E.A., Stewardson, A.J. and Harbarth, S. (2016) ‘Will 10 million people die a year due to antimicrobial resistance by 2050?’, PLOS Medicine, 13(11), p. e1002184 [online]. Available at https://doi.org/10.1371/journal.pmed.1002184 (accessed 10 September 2025).
De Silva, S. and Higgins, A.M. (2023) ‘Clinimetrics: the quality adjusted life year’, Journal of Physiotherapy, 69(1), pp. 58–9 [online]. Available at https://doi.org/10.1016/j.jphys.2022.06.008 (accessed 10 September 2025).
Elliott, T.M., Harris, P.N., Roberts, L.W., Doidge, M., Hurst, T., Hajkowicz, K., Forde, B., Paterson, D.L. and Gordon, L.G. (2021) ‘Cost-effectiveness analysis of whole-genome sequencing during an outbreak of carbapenem-resistant Acinetobacter baumannii’, Antimicrobial Stewardship & Healthcare Epidemiology, 1(1), e62, 13 December [online]. Available at https://www.cambridge.org/core/journals/antimicrobial-stewardship-and-healthcare-epidemiology/article/costeffectiveness-analysis-of-wholegenome-sequencing-during-an-outbreak-of-carbapenemresistant-acinetobacter-baumannii/D7FBAD9D70CEB555A858FE1B302C30B1 (accessed 7 October 2025).
Feikin, D.R., Scott, J.A. and Gessner, B.D. (2014) ‘Use of vaccines as probes to define disease burden’, The Lancet, 383(9930), pp. 1762–70; erratum in The Lancet, 383(9935), p. 2126 [online]. Available at https://doi.org/10.1016/s0140-6736(13)61682-7 (accessed 7 October 2025).
Gebretekle, G.B., Mariam, D.H., Mac, S., Abebe, W., Alemayehu, T., Degu, W.A., Libman, M., Yansouni, C.P., Fenta, T.G., Semret, M. and Sander, B. (2021) ‘Cost–utility analysis of antimicrobial stewardship programme at a tertiary teaching hospital in Ethiopia’, BMJ Open, 11(12), e047515, 17 December [online]. Available at https://pmc.ncbi.nlm.nih.gov/articles/PMC8685939/ (accessed 10 September 2025).
Global Burden of Disease Study 2021 (GBD) (2021) ‘Disability weights’, GHDx [online]. Available at https://ghdx.healthdata.org/record/ihme-data/gbd-2021-disability-weights (accessed 1 June 2025).
Global Laboratory eTools (n.d.) ‘SEDRI LIMS’ [online]. Available at https://www.lab-etools.org/etool/sedri-lims/ (accessed 9 September 2025).
Institute for Health Metrics and Evaluation (IHME) (n.d.) ‘Antimicrobial resistance (AMR)’ [online]. Available at https://www.healthdata.org/research-analysis/health-topics/antimicrobial-resistance-amr-our-approach (accessed 9 September 2025).
Institute for Health Metrics and Evaluation (IHME)/University of Oxford (n.d.) ‘MICROBE’ [online]. Available at https://vizhub.healthdata.org/microbe/ (accessed 9 September 2025).
Kazibwe, J., Gheorghe, A., Wilson, D., Ruiz, F., Chalkidou, K. and Chi, Y.-L. (2022) ‘The use of cost-effectiveness thresholds for evaluating health interventions in low- and middle-income countries from 2015 to 2020: a review’, Value in Health, 25(3), pp. 385–9 [online]. Available at https://doi.org/10.1016/j.jval.2021.08.014 (accessed 10 September 2025).
Martins, S.B., Afonso, J.S., Fastl, C., Huntington, B. and Rushton, J. (2024) ‘The burden of antimicrobial resistance in livestock: a framework to estimate its impact within the Global Burden of Animal Diseases programme’, One Health, 19, pp. 100917–100917 [online]. Available at https://doi.org/10.1016/j.onehlt.2024.100917 (accessed 10 September 2025).
MirkovonHein [YouTube user] (2024) ‘Direct vs. indirect cost in HEOR’, YouTube, 10 November [online]. Available at https://www.youtube.com/shorts/GRj0TnntQLI (accessed 9 September 2025).
Ochalek, J., Lomas, J. and Claxton, K. (2018) ‘Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data’, BMJ Global Health, 3(6) [online]. Available at https://doi.org/doi:10.1136/bmjgh-2018-000964 (accessed 10 September 2025).
Poudel, A.N., Zhu, S., Cooper, N., Little, P., Tarrant, C., Hickman, M. and Yao, G. (2023) ‘The economic burden of antibiotic resistance: a systematic review and meta-analysis’, PLOS One, 8 May [online]. Available at https://doi.org/10.1371/journal.pone.0285170 (accessed 10 September 2025).
Review on Antimicrobial Resistance, https://amr-review.org/ (accessed 1 June 2025).
Rodríguez-Baño, J., López-Prieto, M.D., Portillo, M.M., Retamar, P., Natera, C., Nuño, E., Herrero, M., del Arco, A., Muñoz, A., Téllez, F., Torres-Tortosa, M., Martín-Aspas, A., Arroyo, A., Ruiz, A., Moya,. R., Corzo, J.E., León, L., Pérez-López, J.A. and SAEI/SAMPAC Bacteraemia Group (2010) ‘Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals’, Clinical Microbiology and Infection, 16(9), pp. 1408–13 [online]. Available at https://pubmed.ncbi.nlm.nih.gov/19845694/ (accessed 8 September 2025).
Salinas-Escudero, G., De la Rosa-Zamboni, D., Carrillo-Vega, M.F., Gamiño-Arroyo, A.E., Toledano-Toledano, F., Ortega-Riosvelasco, F., Granados-García, V., Villa-Guillén, M. and Garduño-Espinosa, J. (2023) ‘Cost-effectiveness analysis of a hand hygiene monitoring system in a tertiary pediatric hospital in Mexico’, Frontiers in Public Health, 11:1117680 [online]. Available at https://pmc.ncbi.nlm.nih.gov/articles/PMC10034395/ (accessed 9 September 2025).
Shrestha, P., Cooper, B.S., Coast, J., Oppong, R., Thuy, N.D.T., Phodha, T., Celhay, O., Guerin, P.J., Wertheim, H. and Lubell, Y. (2018) ‘Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use’, Antimicrobial Resistance & Infection Control, 7 [online]. Available at https://doi.org/10.1186/s13756-018-0384-3 (accessed 10 September 2025).
Torgerson, P.R., Rüegg, S., Devleesschauwer, B., Abela-Ridder, B., Havelaar, A.H., Shaw, A.P.M., Rushton, J. and Speybroeck, N. (2018) ‘zDALY: An adjusted indicator to estimate the burden of zoonotic diseases’, One Health, 5, pp. 40–45 [online]. Available at https://doi.org/10.1016/j.onehlt.2017.11.003 (accessed 10 September 2025).
Turner, P., Andrew Fox-Lewis, A., Shrestha, P., Dance, D.A.B., Wangrangsimakul, T., Cusack, T.-P., Ling, C.L., Hopkins, J., Roberts, T., Limmathurotsakul, D., Cooper, B.S., Dunachie, S., Moore, C.E., Dolecek, C., van Doorn, H.R., Guerin, P.J., Day, N.P.J. and Ashley, E.A. (2019) ‘Microbiology Investigation Criteria for Reporting Objectively (MICRO): a framework for the reporting and interpretation of clinical microbiology data’, BMC Medicine, 17(1), p. 70 [online]. Available at https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1301-1 (accessed 7 October 2025).
Turner, H.C., Archer, R.A., Downey, L.E., Isaranuwatchai, W., Chalkidou, K., Jit, M. and Teerawattananon, Y. (2021) ‘An introduction to the main types of economic evaluations used for informing priority setting and resource allocation in healthcare: key features, uses, and limitations’, Frontiers in Public Health, 9, 25 August [online]. Available at https://doi.org/10.3389/fpubh.2021.722927 (accessed 10 September 2025).
Turner, H.C., Sandmann, F.G., Downey, L.E., Orangi, S., Teerawattananon, Y., Vassall, A. and Jit, M. (2023) ‘What are economic costs and when should they be used in health economic studies?’, Cost Effectiveness & Resource Allocation, 21(1), pp. 1–11 [online]. Available at https://doi.org/10.1186/s12962-023-00436-w (accessed 10 September 2025).
United Nations (2016) ‘Political declaration of the high-level meeting of the General Assembly on antimicrobial resistance’, 71st session, agenda item 127, 22 September. New York, NY: UN [online]. Available at https://digitallibrary.un.org/record/842813?ln=en&v=pdf (accessed 10 September 2025).
WHONET, https://whonet.org/ (accessed 9 September 2025).
Woods, B., Revill, P, Sculpher, M. and Claxton, K. (2016) ‘Country-level cost-effectiveness thresholds: Initial estimates and the need for further research’, Value in Health, 19(8), pp. 929–35 [online]. Available at https://doi.org/10.1016/j.jval.2016.02.017 (accessed 10 September 2025).
World Bank Group (2024) ‘Antimicrobial resistance (AMR)’, brief, 2 October [online]. Available at https://www.worldbank.org/en/topic/health/brief/antimicrobial-resistance-amr (accessed 1 June 2025).
World Health Organization (WHO) (2017) Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–17. Geneva: WHO [online]. Available at https://cdn.who.int/media/docs/default-source/searo/amr/global-antimicrobial-resistance-surveillance-system-(glass)-report-early-implementation-2016-2017.pdf (accessed 8 September 2025).
World Health Organization (WHO) (n.d. 1) ‘Countries/areas by WHO region’ [online]. Available at https://ourworldindata.org/grapher/who-regions#:~:text=World%20Health%20Organization%20%E2%80%93%20Countries/areas,20region%2520%2D%252012bfe12.pdf (accessed 9 September 2025).
World Health Organization (WHO) (n.d. 2) ‘GLASS dashboard’ [online]. Available at https://worldhealthorg.shinyapps.io/glass-dashboard/_w_a891c93dd3ad4f328ca95650d995da3d/#!/amr (accessed 9 September 2025).
World Health Organization (WHO) (n.d. 3) ‘Global Antimicrobial Resistance and Use Surveillance System (GLASS)’ [online]. Available at https://www.who.int/initiatives/glass (accessed 9 September 2025).
World Health Organization (WHO) (n.d. 4) ‘Library of AMR national action plans’ [online]. Available at https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans (accessed 8 September 2025).
Further reading
Buckley, G.J. and Palmer, G.H. (eds) (2022) ‘The health and economic burden of resistance’, Chapter 3 in Combating Antimicrobial Resistance and Protecting the Miracle of Modern Medicine. Washington, DC: National Academies Press [online]. Available at https://www.ncbi.nlm.nih.gov/books/NBK577288/ (accessed 10 September 2025).
Love-Koh, J., Walker, S.M., Kataika, E., Sibandze, S., Arnold, M., Ochalek, J., Griffin, S., Revill, P. and Sculpher, M. (2019) Economic Analysis for Health Benefits Package Design, CHE Research Paper 165. York: Centre for Health Economics, University of York [online]. Available at https://eprints.whiterose.ac.uk/id/eprint/147199/1/CHERP165_economic_analysis_HBP.pdf (accessed 10 September 2025).
Mendelson, M., Lewnard, J.A., Sharland, M., Cook, A., Pouwels, K.B., Alimi, Y., Mpundu, M., Wesangula, E., Weese, J.S., Røttingen, J.A. and Laxminarayan, R. (2024) ‘Ensuring progress on sustainable access to effective antibiotics at the 2024 UN General Assembly: a target-based approach’, The Lancet, 403(10443), pp. 2551–64 [online]. Available at https://doi.org/10.1016/S0140-6736(24)01019-5 (accessed 10 September 2025).
Shorr, A.F., Tabak, Y.P., Killian, A.D., Gupta, V., Liu, L.Z. and Kollef, M.H. (2006) ‘Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database’, Critical Care Medicine, 34(10), pp. 2588–95 [online]. Available at https://doi.org/10.1097/01.CCM.0000239121.09533.09 (accessed 10 September 2025).
Vrijens, F., Hulstaert, F., Van de Sande, S., Devriese, S., Morales, I. and Parmentier, Y. (2010) ‘Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs’, Journal of Hospital Infection, 75(3), pp. 158–62 [online]. Available at https://doi.org/10.1016/j.jhin.2009.12.006 (accessed 10 September 2025).
World Health Organization/Food and Agriculture Organization of the United Nations/United Nations Environment Programme/World Organisation for Animal Health (WHO/FAO/UNEP/WOAH) (2023) A One Health Priority Research Agenda for Antimicrobial Resistance. Geneva: WHO [online]. Available at https://www.woah.org/app/uploads/2023/06/one-health-amr-research-prioritisation-launch-v7-2.pdf (accessed 10 September 2025).
Acknowledgements
This free course was collaboratively written by Cherry Lim, Chris Painter and Ilias Kounatidis, and reviewed by Corrado Minetti, Koen Pouwels, Rachel McMullan, Heidi Hopkins and Dan Whitaker.
Grateful acknowledgement is made to the following sources:
Course image: Katka/Pixabay
Figures 1–4: World Health Organization
Figure 5: Planemad, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) via Wikimedia Commons
Every effort has been made to contact copyright holders. If any have been inadvertently overlooked the publishers will be pleased to make the necessary arrangements at the first opportunity.