Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 22 January 2026, 4:41 AM
Immunization Module: Antiviral Vaccines
Study Session 3 Antiviral Vaccines
Introduction
In this study session, you will learn about the antiviral vaccines that are in common use for preventing viral diseases in most developing countries, including Ethiopia, and the recommended dosages and schedules for immunization with these vaccines. There are many other antiviral vaccines available in the world that are not widely used in developing countries, so we will concentrate here on the common antiviral vaccines available for the Expanded Programme on Immunization (EPI) in Ethiopia. These are hepatitis B (HepB) vaccine, oral polio vaccine (OPV) and measles vaccine. You will also be reminded about the need to give vitamin A supplements at the same time as the measles vaccine, and to children who present to you with measles. Then we describe one of the newer antiviral vaccines against rotavirus, which is being added to the EPI. Finally, we mention yellow fever vaccine, which is not in the EPI but may be required for travel abroad.
Studying this session will enable you to give clear information to the people in your community about the value of immunizing their children with these vaccines, which prevent long-term disability and save many millions of lives. You will be able to tell parents that these vaccines are very safe, and any adverse events following immunization are almost always very mild and easily treated. We will also describe the contraindications that mean you should not immunize a child, either temporarily or permanently.
To guarantee long-term protection, all doses of the antiviral EPI vaccines should be given. If a child misses the scheduled date for an immunization, he or she should be given the missed dose as soon as possible. There is no need to re-start the immunization schedule.
Learning Outcomes for Study Session 3
When you have studied this session, you should be able to:
- 3.1 Define and use correctly all of the key words printed in bold. (SAQ 3.1)
- 3.2 Describe the common antiviral vaccines available in the Expanded Programme on Immunization (EPI) in Ethiopia, and the new rotavirus vaccine that will become available soon. (SAQ 3.2)
- 3.3 Describe the storage, dosages and schedules of the common antiviral EPI vaccines, and the dosage of vitamin A given routinely with measles vaccine or to children with measles. (SAQs 3.2, 3.3 and 3.4)
- 3.4 Describe the possible adverse events following immunization with the antiviral EPI vaccines and how you manage them at Health Post level. (SAQ 3.3)
- 3.5 Describe the contraindications for the antiviral EPI vaccines. (SAQ 3.5)
3.1 Hepatitis B vaccine (HepB)
3.1.1 What is HepB vaccine?
Hepatitis B diseases are discussed in Study Session 4 of the Communicable Diseases Module, Part 1.
Hepatitis B (HepB) vaccine protects against the hepatitis B diseases, which affect the liver and are caused by hepatitis B viruses. If a child is infected with hepatitis B viruses, liver disease may develop many years later in adult life. In Ethiopia, HepB vaccine is routinely given to infants as one of the five vaccines combined in the pentavalent vaccine (which also protects against diphtheria, pertussis, tetanus and Haemophilus influenzae type b). The four antibacterial vaccines in the pentavalent vaccine used in Ethiopia were described in Study Session 2.
HepB vaccine is a recombinant vaccine. What does this mean?
A recombinant vaccine is produced by inserting genetic material from a disease-causing infectious agent into harmless cells in the laboratory. The cells with the new genes begin to manufacture the unique antigens that identify the infectious agent. These antigens are then purified and used in the vaccine.
HepB vaccine is also available on its own as a single vaccine, which may be given to healthworkers as a ‘booster’ to increase their protection against infection by bloodborne hepatitis B viruses from patients. The vaccination schedule in adults is not the same as the schedule you already know for infants, who receive HepB vaccine as part of the pentavalent vaccine in the Ethiopian EPI.
How many doses and at what ages should infants receive pentavalent vaccine containing HepB vaccine? What is the route of administration?
They should receive three doses of pentavalent vaccine at the age of 6, 10 and 14 weeks, as you learned in Study Session 2. The route of administration is by intramuscular injection into the left outer upper thigh muscle.
The HepB component of the pentavalent vaccine is very safe and effective: up to 95% of infants who receive all three doses of pentavalent vaccine are protected against hepatitis B virus infection. The contraindications are the same as already described for pentavalent vaccine: infants with a high-grade fever should not be vaccinated until they recover. An infant who develops a severe allergic reaction, swollen lymph glands or encephalopathy to a previous dose of pentavalent vaccine should not be given another dose.
Do you remember the storage conditions for pentavalent vaccine containing HepB vaccine and the four antibacterial vaccines?
The vaccine vials should be stored in a refrigerator at between +2oC and +8oC, and never frozen.
3.2 Oral polio vaccine (OPV)
3.2.1 What is OPV?
You will learn more about the oral administration of vaccines in Study Session 4.
Oral polio vaccine (OPV) is made from live-attenuated polioviruses (note that ‘poliovirus’ is all one word). OPV is a light red or light yellow liquid supplied in vials that either have droppers as caps, or they come with separate glass droppers. The vaccine is given by putting two drops into the child’s mouth (Figure 3.1).

Poliomyelitis is described in Study Session 4 of the Communicable Diseases Module, Part 1. It has been eliminated by vaccination in many countries and the WHO aims to eradicate it from the world.
OPV gives protection against the three types of polioviruses (types 1, 2 and 3) that cause poliomyelitis (polio) — a crippling disease of the brain and spinal cord.
What is the crippling effect of polio called? And what does this mean?
It is called acute flaccid paralysis (AFP), which is the sudden onset of severe weakening or loss of muscle tone in the legs, arms or hands.
Note that the vaccine used for routine immunizations in the EPI is not the only type of OPV available. Other types may be issued to control outbreaks of polio if they occur, but are not used for routine protection of infants; these vaccines should be returned to the central store after the supplementary immunization campaign.
3.2.2 Storage, dosage, schedule and effectiveness of OPV
Store OPV between +2°C and +8°C; but it may be frozen for long-term storage.
Four doses are given, each of two drops. OPV should be given at birth, 6 weeks, 10 weeks and 14 weeks of age. The interval between all doses must be at least four weeks. The birth dose is known as OPV0; the subsequent doses are referred to as OPV1 (at 6 weeks), OPV2 (at 10 weeks), and OPV3 (at 14 weeks).
You should not give OPV0 (the birth dose) to an infant who is more than 14 days old. If this dose has not been given by 14 days, miss this dose and wait until the child is six weeks old, and then give OPV1. You should also give the first doses of the other routine EPI vaccines, including PCV10, at six weeks. The remaining doses should be given as scheduled at 10 and 14 weeks.
If a child spits out the vaccine drops, or vomits immediately after a dose of OPV, it is quite safe to repeat the dose. You should still give the scheduled dose even if a child has diarrhoea at the time; give OPV as usual, but administer an extra (fifth) dose at least four weeks after he or she has received the final dose in the schedule. Ninety-nine per cent of those who are vaccinated with four doses of OPV are protected from polio for life, but during campaigns children are often given additional boosters of OPV to ensure high herd immunity.
3.2.3 Contraindications of OPV and adverse events
OPV is a very safe vaccine and there is no contraindication to prevent giving it. Serious adverse reactions to OPV are very rare: acute flaccid paralysis has been reported in approximately one child in every 1–10 million children who have been vaccinated with OPV. Table 3.1 summarises what you should know about OPV.
| Category | Description |
|---|---|
| Type of vaccine | Live-attenuated antiviral vaccine |
| Number of doses | Four (referred to as OPV0, OPV1, OPV2 and OPV3), plus campaign doses |
| Schedule | At birth, 6, 10 and 14 weeks |
| Additional dose | If the child spits or vomits after OPV, repeat the dose immediately; if the child has diarrhoea, give a fifth dose at least 4 weeks after the scheduled fourth dose |
| Contraindications | None |
| Adverse events | Very rarely AFP; refer immediately to a health centre |
| Special precautions | None |
| Dosage | 2 drops |
| Administration route | Into the mouth (oral) |
| Storage | Store between +2°C and +8°C (may be frozen for long-term storage) |
What should you do if a baby vomits immediately after being given the oral polio vaccine?
You should repeat the dose immediately; it is quite safe to give the baby an extra dose of OPV.
3.3 Measles vaccine
3.3.1 What is measles vaccine?
Measles is discussed in Study Session 4 of the Communicable Diseases Module, Part 1.
Measles vaccine is made from live-attenuated virus and is supplied as a powder that has to be reconstituted with (dissolved in) the special diluent provided to use with it. You will learn about vaccine reconstitution in detail in Study Session 4.
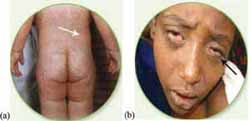
Measles is a highly infectious disease caused by a virus that weakens the immune system, leaving children susceptible to other dangerous childhood infections. The common signs and symptoms of measles are a red skin rash, runny nose and conjunctivitis (Figure 3.2).
Measles vaccine is a live-attenuated vaccine. What does this mean?
You learnt about different types of vaccine in Study Session 1, Section 1.3.
It means it has been made from measles viruses that have been weakened in the laboratory so that they cannot cause the disease. But they are still immunogenic, which means they activate the immune system of the vaccinated person to produce immunity against measles viruses if encountered in the future.
3.3.2 Storage, dosage and schedule of measles vaccine
The vials containing the dry measles vaccine powder can be frozen for long-term storage, but after reconstitution with the correct diluent, measles vaccine should be kept at between +2°C and +8°C, and never frozen. Any remaining reconstituted vaccine must be thrown away after six hours, or at the end of the immunization session, whichever comes first.
One dose of 0.5 ml of measles vaccine is injected subcutaneously (into the fatty layer below the skin and above the muscle) in the outer upper arm as soon as possible after nine months of age. Waiting this long is advisable because the maternal antibodies against measles that are transferred to the unborn baby before birth last longer in the blood of the baby than other antibodies. As a result, immunization with measles vaccine is often not effective before nine months of age. The aim in the EPI in Ethiopia is to give measles vaccine to all children at nine months of age. To achieve high-level population immunity, a second dose is ideally given after 12 months of age during supplementary immunization activities.
What are antibodies and how can maternal antibodies help in combating measles?
You learnt about antibodies in Study Session 1.
Antibodies are specialized proteins that circulate in the blood and other body fluids. They are produced by B cells in the immune system. They identify foreign antigens, such as those found on infectious agents (e.g. the measles virus), and neutralise them so they are unable to cause disease. Maternal antibodies are those produced by the mother when she is vaccinated, which are transmitted passively to the fetus across the placenta, or to her baby in her breastmilk. Maternal antibodies directed against measles viruses will protect the infant from measles in the first few months of his or her life.
In special situations, for instance in measles outbreaks, in emergencies such as in refugee camps with high measles transmission, or among HIV-infected children, an ‘early’ extra dose of measles vaccine may be given at six months. If a child has received measles vaccine before nine months of age, a second dose should be administered at nine months, or as soon as possible afterwards. All children should ideally receive a supplementary dose of measles vaccine after 12 months of age as part of measles elimination campaigns.
3.3.3 Effectiveness of measles vaccine
Measles vaccine is highly effective at preventing measles; 85% of infants who are immunized at 9 months are protected from measles for the rest of their lives; vaccine efficacy rises to 95% after a second dose after 12 months of age. In the year 2000 there were an estimated 750,000 deaths from measles worldwide. By 2008, vaccination campaigns had succeeded in reducing this number by 75%. The World Health Organization (WHO) estimated that in 2008 around 83% of the world’s children were receiving one dose of measles vaccine by their first birthday.
3.3.4 Contraindications for measles vaccine, adverse events and how to manage them
In around 20% of children, a mild fever lasting one to three days may occur approximately one week after immunization. A few children (less than 5%) develop a mild rash. Injection-site abscess or severe allergic reactions including rash, breathing difficulty and fainting occur very rarely. Table 3.2 gives a summary of the possible adverse events following measles vaccination and how to manage them.
| Adverse event | Management | Comments |
|---|---|---|
| Low-grade fever Slight rash | Give paracetamol syrup (5 ml) up to 4 doses | Usually lasts 1 to 3 days |
| Abscess at injection site | Amoxicillin syrup orally three times daily | Refer urgently to a higher health facility |
| Severe rash, breathing difficulty, loss of consciousness | Do not give measles vaccine again | Refer the child to a health centre immediately |
Table 3.3 summarises what you should know about measles vaccine.
| Category | Description |
|---|---|
| Type of vaccine | Live-attenuated antiviral vaccine |
| Number of doses | One in routine EPI schedule, plus one in supplementary campaigns |
| Schedule | At 9 months in the EPI; after 12 months in campaigns |
| Additional early dose | At 6 months in some circumstances (see Section 3.3.2) |
| Contraindications | Severe allergic reaction to previous dose |
| Adverse events | Fever, rash and (rarely) severe allergic reaction or abscess (see Section 3.3.4) |
| Special precautions | None |
| Dosage | 0.5 ml |
| Injection site | Outer upper arm |
| Injection type | Subcutaneous |
| Storage | Store between +2°C and +8°C (Note: the vaccine powder may be frozen for long-term storage, but not the diluent or the reconstituted vaccine) |
3.3.5 Vitamin A supplements and measles prevention
It is important to know that in countries such as Ethiopia where vitamin A deficiency frequently occurs among children, vitamin A should be given routinely every six months. If a child has not previously received a vitamin A supplement, it should be given at the same time as the measles vaccine. This is because measles increases the risk of blindness due to vitamin A deficiency. Signs of vitamin A deficiency are white spots on the sclera (white part of the eye) and clouding of the cornea (the thin tissue covering the black centre of the eye and the coloured parts around it). In severe cases, blindness results.
Vitamin A is supplied in capsules of 100,000 IU (international units, which is the standard measurement for vitamin doses). Each capsule has a nipple at one end. The drops are given by cutting across the middle of the nipple with scissors and immediately squeezing the drops into the child’s mouth (Figure 3.3 on the next page). Vitamin A drops are also given in Child Health Days to ensure the dose is repeated every six months, until the child reaches five years.

The routine dose of vitamin A for a child aged 6–11 months is the drops from one capsule (100,000 IU); the drops from two capsules (200,000 IU) are given to children aged 12–59 months at regular intervals, every six months. This ensures that all children are fully protected from the harmful effects of vitamin A deficiency.
Vitamin A for children with measles
What should you do if you see a child who has already developed measles? Do not give vitamin A drops if the child has received a dose within the last month. But if a child with measles has not recently received a vitamin A supplement, give the vitamin A treatment dosages summarised in Table 3.4. The second dose should be given 24 hours after the first dose.
| Age | Immediately on diagnosis | After 24 hours | Follow-up |
|---|---|---|---|
| Infants less than 6 months old | 50,000 IU | 50,000 IU | Third dose given two to four weeks later if there are still signs of vitamin A deficiency |
| Infants aged 6–11 months | 100,000 IU | 100,000 IU | |
| Children aged 12 months and over | 200,000 IU | 200,000 IU |
3.4 Rotavirus vaccine
Rotavirus vaccine is one of the new antiviral vaccines that should soon become available as part of the routine EPI in Ethiopia. Rotaviruses are the leading cause of severe diarrhoeal disease and dehydration among children in many developed and developing countries. The WHO estimates that 20% of child deaths under five years from diarrhoeal diseases worldwide are due to rotavirus infection — most of them in countries like Ethiopia. Globally, more than 125 million cases and up to 610,000 deaths annually are due to rotavirus diarrhoea.
3.4.1 Dosage and schedule of rotavirus vaccine (RotarixTM)
Two new live-attenuated rotavirus vaccines have been licensed for use in routine immunization programmes. They are both given orally to infants as drops into the mouth. The vaccine chosen for the EPI in Ethiopia is known by its brand name RotarixTM. It is a liquid suspension vaccine, supplied in single-dose ‘squeeze-tube’ vials.
RotarixTM is given in two oral doses, each of 1.5 ml, at the following time intervals:
- First dose at 6 weeks of age, but no later than 12 weeks
- Second dose at least 4 weeks after the first dose
- The two-dose schedule should be completed by 16 weeks, but no later than by 24 weeks of age.
Note that the ideal schedule is to give the first dose of RotarixTM to all infants at 6 weeks of age at the same time as giving Penta1 and OPV1, and give the second dose at 10 weeks at the same time as Penta2 and OPV2.
3.4.2 Storage and effectiveness of RotarixTM
RotarixTM is a freeze-sensitive vaccine that must be stored in the refrigerator at a temperature of between +2oC to +8oC. It is a very safe vaccine, which provides 90–100% protection from severe rotavirus disease to fully immunized infants, and 74–85% protection against rotavirus diarrhoea of any severity.
You will receive instructions on the contraindications for giving rotavirus vaccine, and the management of possible adverse events following immunization, when RotarixTM is introduced into the EPI in Ethiopia.
3.5 Yellow fever vaccine
Finally, we will briefly mention an antiviral vaccine that is not part of the routine EPI in Ethiopia, but is given in certain circumstances. Yellow fever vaccine is a live-attenuated antiviral vaccine, supplied as a powder which must be reconstituted with the appropriate diluent before use. It is not included in the national EPI in Ethiopia because the risk is very low, but it is given to people who want to travel abroad to countries like Kenya and Uganda where yellow fever is endemic. Some countries (like the USA and South Africa) require a current yellow fever vaccination certificate as a condition of entry from Ethiopia. Yellow fever is a viral vector-borne disease, transmitted by certain species of mosquitoes when they take a blood meal. Infection can result in a high fever, nausea and internal bleeding leading to shock, and in extreme cases, death.
Yellow fever vaccine is a live-attenuated vaccine. Which other antiviral vaccines of this type have you already learnt about in this study session?
Measles vaccine and OPV are also live-attenuated vaccines.
Mild reactions to yellow fever vaccine occur occasionally and include headache, muscle pain or low-grade fever.
3.6 In conclusion
Table 3.5 summarises the current and forthcoming antiviral EPI vaccines in Ethiopia.
| Vaccine | Protects against | Doses/when | Route |
|---|---|---|---|
| HepB | Hepatitis B | Three: at 6, 10 and 14 weeks, in the pentavalent vaccine | Intramuscular, upper outer left thigh |
| OPV | Poliomyelitis | Four: at birth, then at 6, 10 and 14 weeks (additional campaign doses may be given) | Oral (drops into the mouth) |
| Measles | Measles | One: at 9 months, or as soon as possible thereafter (plus one additional campaign dose) | Subcutaneous, outer upper arm |
| Rotavirus | Rotavirus diarrhoea | Two: at 6 and 10 weeks (but first dose no later than 12 weeks, second dose no later than 24 weeks, with an interval of at least 4 weeks between doses) | Oral (drops into the mouth) |
Summary of Study Session 3
In Study Session 3, you have learned that:
- The common antiviral vaccines in the EPI in Ethiopia are hepatitis B (HepB) vaccine, which is given in the pentavalent vaccine, oral polio vaccine (OPV) and measles vaccine. A rotavirus vaccine (RotarixTM) to protect against diarrhoea and dehydration caused by rotaviruses will become part of the EPI soon.
- To guarantee long-term protection, all recommended doses of the antiviral vaccines should be given. If a child misses the scheduled date for an immunization, the child should be given the missed dose as soon as possible. There is no need to restart the immunization schedule.
- The antiviral EPI vaccines are very effective and safe for children, and any adverse events following immunization are usually mild and easily managed; serious adverse events are extremely rare.
- There are no contraindications to prevent giving the oral polio vaccine; if a child vomits a dose, give a replacement dose immediately; if the child has diarrhoea at the time of vaccination, give a fifth dose at least 4 weeks after the scheduled fourth dose.
- Contraindications for the other antiviral vaccines are high-grade fever, or a severe allergic reaction or encephalopathy following a previous dose.
- Vitamin A drops should be routinely given at the same time as measles vaccine, or if a child presents with measles, to reduce the risk of eye damage which can be serious enough to cause blindness.
- Yellow fever vaccine is not part of the EPI in Ethiopia, but may be required by travellers going abroad to certain countries.
Self-Assessment Questions (SAQs) for Study Session 3
Now that you have completed this study session you can assess how well you have achieved its Learning Outcomes by answering the following questions. Some of the question test the Learning Outcomes for Study Session 2, as well as those for Study Session 3. Write your answers in your Study Diary and discuss them with your Tutor at the next Study Support Meeting. You can check your answers with the notes on the Self-Assessment Questions at the end of this Module.
SAQ 3.1 (tests Learning Outcomes 2.1, 2.2, 3.1 and 3.2)
Classify each of the following vaccines as either antibacterial or antiviral (or both) by putting crosses in the appropriate columns of Table 3.6. Write the name of the diseases or conditions that each vaccine prevents in the last column of the table.
| Vaccine | Antibacterial | Antiviral | Protects against |
|---|---|---|---|
| BCG vaccine | |||
| Measles vaccine | |||
| Pentavalent vaccine | |||
| Yellow fever vaccine | |||
| Pneumococcal vaccine (PCV10) | |||
| OPV | |||
| TT vaccine | |||
| Meningococcal vaccine | |||
| Rotavirus vaccine |
Answer
The completed version of Table 3.6 appears below.
| Vaccine | Antibacterial | Antiviral | Protects against |
|---|---|---|---|
| BCG vaccine | X | Tuberculosis | |
| Measles vaccine | X | Measles | |
| Pentavalent vaccine | X (four components) | X (one component) | Diphtheria, pertussis, tetanus, Haemophilus influenzae type b meningitis and pneumonia, hepatitis B liver diseases |
| Yellow fever vaccine | X | Yellow fever | |
| Pneumococcal vaccine (PCV10) | X | Pneumococcal diseases (including pneumonia) | |
| OPV | X | Poliomyelitis | |
| TT vaccine | X | Neonatal tetanus (and tetanus in women) | |
| Meningococcal vaccine | X | Meningococcal meningitis | |
| Rotavirus vaccine | X | Diarrhoea and dehydration caused by rotaviruses |
SAQ 3.2 (tests Learning Outcome 3.3)
Summarise the dosage, route of administration and schedule for each of the current antiviral EPI vaccines used in Ethiopia by completing Table 3.7.
| Vaccine | Dosage and route | Schedule |
|---|---|---|
| HepB (as part of pentavalent vaccine) | ||
| OPV | ||
| Measles |
Answer
The completed version of Table 3.7 appears below.
| Vaccine | Dosage and route | Schedule |
|---|---|---|
| HepB (as part of pentavalent vaccine) | 0.5 ml, three intramuscular injections | At 6, 10 and 14 weeks |
| OPV | 2 drops, four oral doses | At birth, and 6, 10 and 14 weeks |
| Measles | 0.5 ml, one subcutaneous injection in the EPI, plus one campaign dose | At 9 months of age in the EPI; campaign dose after 12 months |
SAQ 3.3 (tests Learning Outcome 3.4)
Complete Table 3.8 to indicate the possible adverse events following immunization with OPV and measles vaccines, and their management at Health Post level.
| Vaccine | Adverse events | Management |
|---|---|---|
| OPV | ||
| Measles |
Answer
The completed version of Table 3.8 appears below.
| Vaccine | Adverse events | Management |
|---|---|---|
| OPV | None | None required |
| Measles | Mild rash Mild fever | None required Paracetamol syrup |
SAQ 3.4 (tests Learning Outcomes 2.3 and 3.3)
What immunizations (if any) should the following children be given? Assume that rotavirus vaccine has not yet been introduced.
- a.A newborn baby.
- b.A ten-month-old child who has had BCG, OPV3, PCV3 and Penta3.
- c.An eight-month-old child who has had BCG, OPV3, PCV3 and Penta3.
- d.A six-week-old child who has had BCG and OPV0.
Answer
The immunizations (if any) that these individuals should receive are as follows:
- a.A newborn baby should be given BCG and OPV0.
- b.A ten-month-old child who has had BCG, OPV3, PCV3 and Penta3 should be given measles vaccine.
- c.An eight-month-old child who has had BCG, OPV3, PCV3 and Penta3 should not be given any further vaccinations until he or she is 9 months old, when measles vaccine should be given.
- d.A six-week-old child who has had BCG and OPV0 should be given OPV1, PCV1 and Penta1.
SAQ 3.5 (tests Learning Outcomes 2.5 and 3.5)
Fatima’s grandmother brings her to your Health Post when she is ten weeks old for OPV2, PCV2 and Penta2. Three days later, Fatima becomes very ill and develops a severe allergic reaction. After a brief period of hospitalisation, she recovers fully. When Fatima is 14 weeks old, her grandmother brings her to see you at an immunization clinic. She has a mild episode of diarrhoea at the time.
- a.What vaccines (if any) should you give Fatima?
- b.What should you explain to Fatima’s grandmother?
Answer
- a.Immunize Fatima with OPV3; it is safe to give this vaccine even though she has mild diarrhoea. But do not give her PCV3 or Penta3 because she developed a severe allergic reaction three days after the earlier immunization, which may have been an adverse vaccine reaction following immunization with one of these vaccines. It is very unlikely to have been due to the previous dose of OPV.
- b.Explain to the grandmother that it is safe for Fatima to have another dose of OPV, and why you are not giving the child another dose of the other two vaccines. Tell the grandmother to come back after another four weeks; because of the diarrhoea Fatima has today, she will need an extra (fifth) dose of OPV in four weeks’ time.