Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 8 March 2026, 5:11 AM
Immunization Module: Antibacterial Vaccines
Study Session 2 Antibacterial Vaccines
Introduction
You learned about the main vaccine-preventable bacterial diseases in Study Session 3 of the Communicable Diseases Module, Part
The most effective way of reducing the major vaccine-preventable diseases is to maintain a high level of immunization in the whole population, using the routine Expanded Programme on Immunization (EPI) vaccines. In this study session, you will learn about the antibacterial vaccines that are approved for routine use in Ethiopia for preventing some common bacterial diseases, and the storage, dosages and schedules for immunization with these vaccines.
This study session will also help you to give clear information about vaccination to the people in your community, including the importance of giving the vaccines as scheduled to their children – even though it makes them cry for a short time (Figure 2.1). Reassure parents about the low risk of adverse events following immunization (AEFIs) and how to manage vaccine reactions if they occur.
As you learned in Study Session 1, contraindication — in the context of immunization — means a medical reason for not giving the vaccine, either temporarily or permanently. Note that children with a mild illness should still be immunized at the scheduled time. Contraindications, such as a high-grade fever (38.5oC or above), mean you should refer the child to a health centre and wait until they recover before giving the missed vaccine dose. Specific contraindications for each vaccine are given in the sections that follow.
Learning Outcomes for Study Session 2
When you have studied this session, you should be able to:
- 2.1 Define and use correctly all of the key words printed in bold. (SAQs 2.1 and 2.5)
- 2.2 Describe the antibacterial vaccines included in the Expanded Programme on Immunization (EPI) in Ethiopia. (SAQs 2.1, 2.2 and 2.4)
- 2.3 Describe the storage, dosages and schedules of the antibacterial EPI vaccines. (SAQs 2.1 and 2.2)
- 2.4 Describe the possible adverse events following immunization with the antibacterial EPI vaccines and how you manage them at Health Post level. (SAQs 2.3 and 2.4)
- 2.5 Describe the contraindications that mean you should not immunize a child with one of the antibacterial EPI vaccines. (SAQs 2.3 and 2.5)
2.1 BCG vaccine
2.1.1 What is BCG?
Tuberculosis diagnosis and treatment is fully described in Study Sessions 13 to 17 in the Communicable Diseases Module, Part 2.
You have already learned about the different types of vaccines in Study Session 1. BCG is a live-attenuated antibacterial vaccine that protects against severe forms of tuberculosis in infants and young children. Tuberculosis (TB) is a disease caused by the bacterium Mycobacterium tuberculosis. It usually attacks the lungs, but can also affect other parts of the body, including the bones, joints and brain. The letters, B, C and G stand for Bacillus of Calmette and Guerin.
Bacillus describes the rod shape of the tuberculosis bacteria; Calmette and Guerin are the names of the people who developed the vaccine.
What does a live-attenuated antibacterial vaccine mean?
Bacteria in the vaccine are alive, but they have been weakened (attenuated) in the laboratory so that they cannot cause the disease.
2.1.2 Effectiveness of BCG vaccine
The effectiveness of a vaccine means its capability to protect vaccinated people from the disease that immunization aims to prevent. In the case of BCG vaccine, the effectiveness is highly variable — protection from TB ranges from 0–80% of those who are vaccinated. This means that some vaccinated individuals may not be protected against TB at all, and up to 80% of the others will receive some protection. The strongest reason for vaccinating with BCG is that it protects young children against developing the more serious forms of tuberculosis, such as TB meningitis (affecting the brain), TB in both lungs and extra-pulmonary TB (affecting other organs).
2.1.3 BCG vaccine storage, dosage and immunization schedule
BCG vaccine is a freeze-dried powder, supplied in small glass bottles called ampoules. Vaccines that come as powders must be mixed with a liquid called a diluent. This process is known as reconstitution, which means thoroughly mixing the powder with the diluent to activate the vaccine before it can be administered. Before use, you must reconstitute BCG vaccine powder with the appropriate diluent supplied for this purpose, using a mixing syringe to mix the diluent with the powder in the ampoule. You will learn how to do this in Study Session 4.
BCG storage
BCG vaccine can be also affected and damaged by heat and light, so it should be stored between +2°C and +8°C. The vaccine powder may be frozen for long-term storage, but the diluent and the reconstituted vaccine must never be frozen. Since BCG vaccine is easily destroyed by sunlight, the vials containing the vaccine powder are mostly made from black or brown glass. Some other vaccines which are supplied as powders can stay in good condition for a long time in a refrigerator and are not spoiled as long as they remain dry. But BCG vaccine can be spoiled and lose its strength in a very short time. Also, since other harmful bacteria can grow in the reconstituted BCG vaccine, it should be used within six hours of mixing the powder with the diluent. If there is any leftover reconstituted BCG vaccine, it should be thrown away in the correct medical waste container at the end of the immunization session.
BCG dosage and schedule
The recommended injection dose for newborns and infants under one year is 0.05 ml, containing 0.05 mg of BCG vaccine powder which has been reconstituted with 0.05 ml of the specific diluent supplied with the vaccine. For children aged over one year, the dose is twice this amount — 0.1 ml containing 0.1 mg of BCG vaccine powder.
How to give an intradermal injection and other vaccination routes are described in detail in Study Session 4 of this Module.
BCG vaccine is given at birth or as soon as possible thereafter. The vaccine is given intradermally (into the top layer of the skin) in the right upper arm in Ethiopia. This is so the injection site can be inspected later.
Table 2.1 summarises the characteristics of the BCG vaccine. The only reason for not giving the BCG vaccine is if the newborn has symptoms of HIV-disease, which may include a high-grade fever. You will learn more about routes of administration and cold storage of all the common vaccines, including BCG, in Study Sessions 4 and 6 of this Module.
| Category | Description |
|---|---|
| Type of vaccine | Live-attenuated antibacterial vaccine |
| Number of doses | One |
| Schedule | At birth, or as soon as possible after birth. If not given at birth, it is better to give within the first three months, when the infant is at greatest risk of developing the most severe forms of TB, such as TB meningitis. Immunization is generally ineffective at older ages. |
| Booster (additional dose) | None |
| Contraindications | Babies or infants showing symptoms of HIV infection |
| Adverse events | Study Session 7 discusses AEFIs, adverse events following immunization, more generally. Mild normal reaction (swelling, small sore). Rarely, severe reaction, e.g. local abscess, or swelling of glands (lymph nodes) |
| Special precautions | Correct intradermal administration is essential. A special syringe and needle is used for the administration of BCG vaccine (described in Study Session 4) |
| Dosage | BCG vaccine is given in the right arm in Ethiopia, but in the left arm in some other countries. Infants aged under one year, give 0.05 mg of BCG vaccine powder reconstituted in 0.05 ml of diluent; over one year, give 0.01 mg in 0.1 ml of diluent |
| Injection site | Outer upper right arm or shoulder. Routes of administration are described in Study Session 4 |
| Injection type | Intradermal (into the top layer of skin). Types of injection are described in Study Session 4 |
| Storage | Store between +2°C and +8°C. BCG vaccine powder may be frozen for long-term storage, but the diluent and reconstituted vaccine must never be frozen. Discard any reconstituted vaccine after no more than six hours. Vaccine storage is described in Study Session 6 |
2.1.4 Adverse events following BCG immunization and how to treat them
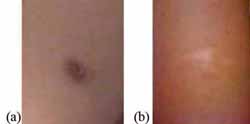
Adverse events may occasionally occur after immunization, in addition to the desired protective effect. They can include swelling and tenderness at the injection site. For BCG immunization, the normal reaction is a small raised swelling, which immediately appears at the injection site (Figure 2.2 on the next page). This usually disappears within 30 minutes.
After approximately two weeks, a small red sore normally develops at the injection site, which is about 10 mm in diameter (the size of the unsharpened end of a pencil). The sore remains for about another two weeks and then heals, leaving a small scar about 5 mm across (Figure 2.3). This is a sign that the child has been effectively immunized. If there is no scar at the injection site six weeks after a BCG immunization, the injection must be repeated. If there is still no skin reaction to the second injection, the child should be referred to a higher-level health facility.
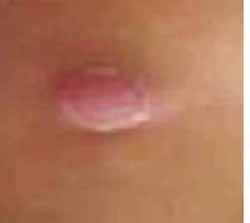
Occasionally, there is an abnormal adverse event following BCG immunization, such as swelling of glands in the armpit, or rarely the formation of an abscess at the injection site (Figure 2.4).
An abscess is a collection of pus and inflamed tissue at the site of bacterial infection. It can be due to bacterial causes other than BCG.
Abnormal adverse events following BCG vaccination may occur because:
- an unsterile needle or syringe was used
- too much vaccine was injected
- the vaccine was injected too deeply under the skin, instead of into its top layer.
Table 2.2 summarises the adverse events that may occur following BCG immunization and how you can treat them at Health Post level.
| Adverse events | Management | Comments |
|---|---|---|
| Small sore at the site of injection after two weeks, which may last two weeks | Keep dry and clean (do not put any ointment on the sore or give the child any medicine) | Will heal naturally to leave a small scar |
| Swollen glands (lymph nodes) in the armpit | Surgical or drug treatment may occasionally be required at a health centre | Refer the child to a health centre |
| Abscess at the injection site | Amoxicillin syrup is an antibiotic preparation used to treat bacterial infections, which is available at Health Post level.daily Amoxicillin syrup orally three times | Refer the child urgently to the next higher health facility |
2.1.5 Who should not get BCG vaccine?
The only contraindications for BCG immunization are symptoms of HIV infection. These include chronic lung infection, tuberculosis, persistent diarrhoea and other serious symptoms of HIV-related diseases, as described in the Communicable Diseases Module, Part 3.
2.2 Pentavalent vaccine
2.2.1 What is pentavalent vaccine?
A vaccine that contains five different antigens in one combined preparation is called a pentavalent vaccine (‘penta’ comes from the Greek word for five). You do not need to remember these details, but the pentavalent vaccine in common use in Ethiopia is a combination of one inactivated whole-cell vaccine (against pertussis bacteria), two sub-unit vaccines (the diphtheria and tetanus toxoids), one conjugate vaccine (against Haemophilus influenza type b bacteria) and one recombinant vaccine (against hepatitis B virus). Thus, the pentavalent vaccine used in Ethiopia in the EPI combines five different vaccines in one injection to protect against four bacterial diseases: diphtheria, tetanus, pertussis and Haemophilus influenzae type b (often abbreviated to Hib), and one viral disease caused by hepatitis B viruses. It is a fully liquid vaccine, which comes in a single dose vial.
Sometimes you will see the pentavalent vaccine used in Ethiopia described as DPT-HepB-Hib vaccine. This is the term used in the IMNCI Module in this curriculum.
What is an inactivated antibacterial vaccine?
It consists of bacteria that have been killed so that they cannot cause the disease.
What is a toxoid?
It is a modified version of the toxin (harmful protein) produced by certain bacteria, including those causing diphtheria and tetanus. The toxoid is used in vaccines to immunize against the disease.
2.2.2 The anti-bacterial components of pentavalent vaccine
Three of the antibacterial components in the pentavalent vaccine used routinely in the EPI in Ethiopia are known as DPT (diphtheria-pertussis-tetanus), referring to the three bacterial diseases they prevent. In Ethiopia, DPT is only given in the pentavalent vaccine, but some other countries give DPT as a separate injection. The fourth antibacterial component of the Ethiopian pentavalent vaccine is called Hib, which stands for Haemophilus influenzae type b. These four components are described below.
Diphtheria toxoid
Diphtheria toxoid is a sub-unit antibacterial vaccine, made from the modified toxin (poison) produced by the bacteria Corynebacterium diphtheriae. The vaccine protects against diphtheria, a serious bacterial infection causing a sore throat, high fever and serious complications which can be fatal. It has become rare in Ethiopia and most other countries where infants are routinely vaccinated against it.
Pertussis vaccine
Pertussis vaccine is an inactivated antibacterial vaccine, which contains killed whole bacterial cells. It protects against pertussis (also known as whooping cough), a highly contagious, acute respiratory infection caused by the bacteria Bordetella pertussis.
Tetanus toxoid (TT)
Tetanus toxoid (TT) is a sub-unit antibacterial vaccine, made from the modified toxin produced by the bacteria Clostridium tetani. Tetanus is a disease that is acquired through exposure to the spores of these bacteria, which are universally present in the soil. TT vaccine is also given on its own as a ‘booster’ to women of childbearing age, and we will say more about this in Section 2.4.
Haemophilus influenzae type b (Hib)
Hib vaccine is a conjugate antibacterial vaccine, which protects against pneumonia and meningitis caused by the bacteria Haemophilus influenzae type b. These bacteria are the most common cause of bacterial meningitis in children under five years of age in countries where Hib vaccine is not routinely given to all infants. Meningitis refers to severe infection of the membranes surrounding the brain and spinal cord, which can rapidly lead to high fever, paralysis and death. Haemophilus influenzae type b bacteria are also the second most common cause of bacterial pneumonia in children. (In Section 2.3 you will learn about the vaccine that protects against Streptococcus pneumoniae, the most common cause of bacterial pneumonia in children.) Pneumonia is a severe infection in the lungs, which causes the air sacs to fill with fluid and pus; this makes breathing painful and difficult, and reduces the oxygen getting into the body. Note that Hib vaccine only protects against diseases caused by type b Haemophilus influenzae bacteria (there are other types), and it does not prevent pneumonia or meningitis caused by other infectious agents.
The fifth component of the pentavalent vaccine used in the EPI in Ethiopia protects against the viruses that cause hepatitis B liver disease; we will describe it in Study Session 3, together with the other antiviral vaccines in the Ethiopian EPI.
2.2.3 Storage, dosage and schedule of pentavalent vaccine
Pentavalent vaccine comes in single-dose glass bottles called vials, which should be stored at between +2°C and +8°C. It should never be frozen, or allowed to become warmer than +8°C, as this will destroy its effectiveness.
If it is allowed to stand for a long time, fine particles settle to the bottom of the vial leaving a cloudy liquid above them. This is normal. Shake the vial to mix the vaccine with the liquid before using it.
Three doses of 0.5 ml each are given intramuscularly (IM, into the muscle) of the upper outer part of the left thigh, before injecting pneumococcal vaccine (PCV10) in the right thigh (see Section 2.3.1). The injections are given to infants at the age of 6, 10 and 14 weeks. If an infant misses a scheduled dose, give it as soon as possible and complete the series of three doses — there is no need to start the series of doses over again. Pentavalent vaccine is usually not given after 6 years of age because there is an increased risk of adverse reactions in older children.
The characteristics of immunization with pentavalent vaccine are summarised in Table 2.3.
| Category | Description |
|---|---|
| Type of vaccine | Five different antigens combined, including one inactivated whole-cell vaccine, two sub-unit vaccines (toxoids), one conjugate vaccine and one recombinant vaccine |
| Number of doses | Three (referred to as Penta1, Penta2 and Penta3) |
| Schedule | At 6, 10 and 14 weeks of age |
| Booster (additional doses) | None in males; boosters of tetanus toxoid vaccine are given to women of childbearing age |
| Contraindications | Severe allergic reaction or encephalopathy to a previous pentavalent immunization (see Section 2.2.5) |
| Adverse events | Mild local reactions are common (see Section 2.2.5); rarely, injection-site abscess |
| Special precautions | Usually not given after six years of age because of the increased risk of serious adverse reactions |
| Dosage | 0.5 ml |
| Injection site | Upper outer left thigh. Injection sites are described in Study Session 4 |
| Injection type | Intramuscular (IM). Types of injection are described in Study Session 4 |
| Storage | Store between +2°C and +8°C. Never freeze. Storage of vaccines is described in Study Session 6 |
2.2.4 Effectiveness of pentavalent vaccine
On average, 78–95% of individuals who have received three vaccine doses will normally be protected against all five diseases covered by the pentavalent vaccine. This means that in every 100 people who receive three doses, on average, between 78–95 of these individuals will be protected against all five diseases.
2.2.5 Adverse events following pentavalent immunization and how to treat them
The possible adverse events following immunization with pentavalent vaccine are generally mild: serious reactions are very rare (see Section 2.2.6). The mild reactions are:
- Soreness. Some children may develop mild soreness, redness, or swelling at the injection site, but this will go away within 1–3 days.
- Fever. Some children may develop a mild fever (a temperature of around 37.3oC to 38.4oC, measured with a thermometer in the child’s armpit, is termed a low-grade fever). It should disappear within a day. Fever that begins more than 24 hours after a pentavalent injection is unlikely to be a reaction to the vaccine and should be investigated.
- Crying for more than three hours, mostly because of pain, occurs in up to 1% of infants.
A serious but rare adverse event is an abscess, which may develop a week or more after immunization (Figure 2.5), usually because an unsterile needle or syringe was used, or the vaccine was not correctly injected into the muscle. The management of these adverse events at Health Post level is summarised in Table 2.4.
| Adverse event | Management | Comments |
|---|---|---|
| Low-grade fever (37.3oC to 38.4oC) | Paracetamol syrup, 5 ml as required, up to a maximum of four doses | Will usually disappear within a day |
| Pain and soreness at the injection site | Paracetamol as above; warm compress (a warm cloth or another warm material) applied under pressure to the sore area of skin and held in place for some time | Will usually disappear within a day |
| Abscess at the injection site | Amoxicillin syrup orally three times daily | Refer the child urgently to the next higher health facility |
2.2.6 Who should not get pentavalent vaccine?
A child who develops a severe reaction to the first vaccination should be referred to a higher level health facility immediately.
Do not give another dose of the pentavalent vaccine if a child develops any of these severe reactions to the first dose:
- Severe allergic reaction (doctors call it anaphylaxis), which includes severe rash, breathing difficulty, signs of shock, weak and rapid pulse, dizziness or fainting.
- Encephalopathy, (Enchephalopathy is pronounced ‘en-seff-ah-lopp-ah-thee’) which is a disease of the brain that presents with coma, decreased level of consciousness and/or prolonged convulsions, occuring within seven days of a pentavalent vaccination, and where the symptoms are not due to another identifiable cause.
2.3 Pneumococcal vaccine (PCV10)
Next we describe a new antibacterial vaccine that is being introduced into the routine EPI in Ethiopia.
Pneumococcal infections are described in the Communicable Diseases Module, Part 4, Study Session 35.
Pneumococcal vaccines (PCVs) protect against pneumonia and other pneumococcal infections caused by Streptococcus pneumoniae bacteria. These bacteria can attack different parts of the body, causing serious infections in the lungs (pneumonia), the inner ear (acute otitis media), the bloodstream (bacteraemia), and the membranes covering the brain and spinal cord (meningitis). The WHO estimates that up to one million children die of pneumococcal infections every year, mainly in sub-Saharan Africa and South East Asia. In Ethiopia, pneumonia is the leading cause of death among children under five years, accounting for 28% of all deaths in this age group.
The Streptococcus pneumoniae bacteria exist in many different ‘strains’. Several different conjugate pneumococcal vaccines have been developed to give protection against different subsets of these strains. The vaccine that is being introduced in Ethiopia as part of the EPI is called PCV10, also known by its brand name Synflorix. PCV10 is highly effective at preventing infections caused by the strains of Streptococcus pneumoniae bacteria included in the vaccine preparation.
2.3.1 Storage, dosage and schedule of PCV10
PCV10 is a freeze-sensitive vaccine, which must be stored in the refrigerator between +2oC to +8oC. You should keep the vials on the same shelf of the refrigerator as the vials of pentavalent vaccine because they are given to infants at the same immunization session. The liquid PCV10 does not contain any preservative, so once you have opened a vial, any unused vaccine should be discarded after six hours and not returned to the refrigerator.
The vaccination schedule for PCV10 (Synflorix) is the same as for the pentavalent vaccine: three doses are given at 6, 10 and 14 weeks of age by intramuscular (IM) injection into the right outer upper thigh muscle (the opposite thigh to the pentavalent vaccine). The dosage for each vaccination with PCV10 is 0.5 ml. The vaccine is a liquid that comes in two-dose vials.
Any child who presents to a health facility for vaccination who is aged less than one year old is eligible to receive PCV10 vaccine if they missed the primary series of doses at 6, 10 and 14 weeks. Children should receive all three doses of PCV10 by their first birthday, but if a child starts their first dose of PCV10 at the age of 11 months, they should complete the remaining two doses at intervals of four weeks. Table 2.5 (on the next page) shows the schedule for PCV10 immunizations for children who have already started the series of pentavalent vaccine doses.
| Vaccination status if infant aged less than one year at time of PCV10 introduction | At first contact | Subsequent contact – after 4 weeks | Subsequent contact – after 4 more weeks |
|---|---|---|---|
| Never received any Penta | Penta1 + PCV1 | Penta2 + PCV2 | Penta3 + PCV3 |
| Already received Penta1 | Penta2 + PCV1 | Penta3 + PCV2 | PCV3 |
| Already received Penta2 | Penta3 + PCV1 | PCV2 | PCV3 |
| Already received Penta3 | PCV1 | PCV2 | PCV3 |
2.3.2 Adverse events and contraindications of PCV10
Refer all cases of severe vaccine reactions urgently.
Mild local reactions (redness, pain and slight swelling at the injection site) and/or mild fever and irritability of the child may occur in one-third to one-half of the infants vaccinated with PCV10, but these reactions usually disappear within 24 hours. Manage these mild reactions as described earlier in Table 2.4 for pentavalent vaccine. Rare severe reactions to the vaccine include convulsions, severe allergic reaction (anaphylaxis), swollen lymph glands and encephalitis.
The contraindications for PCV10 are the same as for pentavalent vaccine: do not give PCV10 to an infant who comes to you with a high-grade fever, or who developed a severe vaccine reaction after a previous dose. However, you should vaccinate infants with PCV10 if they only have a mild illness, such as a common cold or low-grade fever.
2.4 Tetanus toxoid (TT) vaccine
2.4.1 What is tetanus toxoid vaccine?
The same tetanus toxoid (TT) vaccine that is given to infants in the pentavalent vaccine is also given on its own, as a single vaccine, to women of childbearing age (Figure 2.6). The vaccine is a cloudy liquid, and the powder can settle to the bottom of the vial if it is left to stand for a long time. Shake the vial to mix the vaccine powder and liquid before use.

From your reading of Study Session 1, how do you think immunizing women against tetanus also prevents tetanus in their newborn babies?
Neonatal tetanus is a major cause of death in newborns; it is described in detail in Study Session 3 of the Communicable Diseases Module, Part 1.
The antibodies that form in the mother’s body in response to the vaccine pass across the placenta and get into the fetus; they give ‘passive’ protection against tetanus to the baby during the birth and for the first few months of life.
2.4.2 Schedule, dosage, storage and effectiveness of TT vaccine
If given as a separate vaccine to pregnant and non-pregnant women of childbearing age, at least two doses of 0.5 ml of TT vaccine are given intramuscularly (IM) into the upper arm (Figure 2.7); but for maximum long-lasting protection throughout the childbearing years women should receive more than two doses (TT2+), and the ideal is to give five doses. It should be stored at between +2oC and +8oC and never frozen. Table 2.6 summarises the characteristics of TT vaccine; the periods of protection after each dose are given in Table 2.7.

| Category | Description |
|---|---|
| Type of vaccine | Toxoid (sub-unit vaccine) |
| Number of doses | Women: at least two doses – ideally five (see Table 2.7) |
| Schedule | Women: first dose at first contact during childbearing years, or as early as possible in pregnancy (then see Table 2.7) |
| Booster | Every 10 years during childbearing years |
| Contraindications | Severe allergic reaction to a previous dose, or encephalopathy |
| Adverse events | Paracetamol can be given to treat mild reactions, but avoid giving any medication to pregnant women. Mild reactions, e.g. low-grade fever, soreness, redness and pain at the injection site: usually disappears after 1–3 days. |
| Dosage | 0.5 ml |
| Injection site | Women: outer upper arm |
| Injection type | Intramuscular (IM) |
| Storage | Store between +2°C and +8°C. Never freeze |
| Dose (0.5ml) | When given | Duration of protection |
|---|---|---|
| TT1 | At first contact with women of childbearing age, or as early as possible in the pregnancy | No protection |
| TT2 | At least 4 weeks after TT1 | 3 years |
| TT3 | At least 6 months after TT2 | 5 years |
| TT4 | At least 1 year after TT3 | 10 years |
| TT5 | At least 1 year after TT4 | All childbearing years |
2.4.3 Adverse events and contraindications of TT vaccine
The possible adverse events following immunization of women with TT vaccine are usually mild: low-grade fever, and soreness/pain at the injection site (see Table 2.6 above), which can be treated with paracetamol in women who are not already pregnant. Women of child-bearing age who developed a severe allergic reaction or encephalopathy to a previous dose of TT vaccine should not be given TT again.
2.5 Meningococcal vaccines
Finally, we refer to the meningococcal vaccines which are in common use in some countries. Although not yet routinely available in the EPI in Ethiopia, these vaccines are used to control outbreaks of meningitis caused by the Neisseria meningitides bacteria, which occur in at least six different types. Types A, B, C, Y, W135 and X cause most cases of meningococcal meningitis. Quadrivalent vaccines (containing the antigens of four of these types) are available in the USA, and a vaccine specifically designed for Africa is being used in the countries with the highest burden of meningococcal meningitis.
2.6 In conclusion
Table 2.8 summarises the antibacterial vaccines in the Expanded Programme on Immunization (EPI) in Ethiopia. In Study Session 3, we will turn our attention to the antiviral vaccines in the EPI.
| Vaccine | Protects against | Doses/when | Route |
|---|---|---|---|
| BCG | Tuberculosis | One: at or soon after birth | Intradermal, outer upper right arm |
| Pentavalent vaccine | Diphtheria, pertussis, tetanus, Hib disease and hepatitis B | Three: at 6, 10 and 14 weeks | Intramuscular, outer upper left thigh |
| TT | Tetanus | At least two: ideally three to five in women of childbearing age | Intramuscular, outer upper arm |
| PCV10 | Pneumonia caused by some Streptococcus pneumoniae strains | Three: at 6, 10 and 14 weeks | Intramuscular, outer upper right thigh |
Summary of Study Session 2
In Study Session 2, you have learned that:
- The most effective way to protect people from the common vaccine-preventable diseases is to maintain a high level of immunization with the vaccines in the routine Expanded Programme on Immunization (EPI).
- The common EPI antibacterial vaccines used in Ethiopia are: BCG, which protects children against the most serious effects of tuberculosis (TB); a pentavalent vaccine, which protects against diphtheria, pertussis, tetanus, cases of meningitis and pneumonia caused by Haemophilus influenzae type b, and hepatitis B liver disease; and PCV10, which protects against pneumonia and some other infections caused by specific strains of Streptococcus pneumoniae bacteria.
- At least two doses of TT vaccine (and ideally five) should also be given to pregnant women and women of childbearing age to protect them and their newborns from tetanus.
- A meningococcal vaccine is available to control outbreaks of meningitis caused by Neisseria meningitides bacteria; it is not a routine EPI vaccine.
- To guarantee long-term protection, all doses of the routine antibacterial vaccines in the EPI should be given. A child who misses a vaccination should be given the missed dose as soon as possible and complete any remaining doses at the scheduled time.
- The EPI antibacterial vaccines are very effective and safe for infants, and TT vaccine is safe for women of childbearing age and during pregnancy. Adverse events following immunization are usually mild, e.g. low-grade fever and soreness at the injection site, which can easily be managed; serious adverse events are extremely rare.
- Infants with minor illnesses may be immunized safely. But if they are suffering from a high fever (38.5oC or above), they should be referred to a health centre; delay the immunization until after they recover. Other contraindications are HIV disease, severe allergic reaction or encephalopathy following a previous dose of a vaccine.
Self-Assessment Questions (SAQs) for Study Session 2
Now that you have completed this study session, you can assess how well you have achieved its Learning Outcomes by answering these questions. Write your answers in your Study Diary and discuss them with your Tutor at the next Study Support Meeting. You can check your answers with the Notes on the Self-Assessment Questions at the end of this Module.
SAQ 2.1 (tests Learning Outcomes 2.1, 2.2 and 2.3)
Which of the following statements is false? In each case, say what is incorrect.
- A All the antibacterial vaccines in the EPI in Ethiopia are injected intramuscularly in the outer upper arm.
- B All of the antibacterial EPI vaccines will lose their effectiveness if they become frozen.
- C If five doses of TT vaccine are given to a woman of childbearing age at the correct intervals, she will receive the fifth dose at least 2 years and 7 months after the first dose.
- D Most infants who are immunized with three doses of pentavalent vaccine at the correct intervals will be protected against diphtheria, pertussis and tetanus.
- E BCG vaccine is supplied as a powder which has to be mixed with a special diluent to activate the vaccine before use.
- F If a vial of pentavalent vaccine has a deposit like fine sand at the bottom, you should throw it away.
Answer
- A is false. BCG is injected intradermally (into the top layer of the skin). The other antibacterial vaccines in the EPI are injected intramuscularly, but only BCG and TT vaccine are injected in the upper arm. Pentavalent vaccine and PCV10 are injected in the upper outer thigh.
- B is false. BCG vaccine is a powder which can be frozen before it is reconstituted with diluent. The BCG diluent and all the other antibacterial EPI vaccines will not be effective if they become frozen.
- C is true. If five doses of TT vaccine are given to a woman of childbearing age at the correct intervals, she will receive the fifth dose at least 2 years and 7 months after the first dose. The intervals are TT1, then at least 4 weeks to TT2, then at least 6 months to TT3, then at least 1 year to TT4, and then at least one more year to TT5.
- D is true. Between 78% and 95% of infants who are immunized with three doses of pentavalent vaccine at the correct intervals will be protected against pertussis, diphtheria and tetanus. (They will also be protected against hepatitis B and Hib diseases.)
- E is true. BCG vaccine is supplied as a powder which has to be mixed with a special diluent to activate the vaccine before use.
- F is false. If a vial of pentavalent vaccine has a deposit like fine sand at the bottom, it does not need to be thrown away; you should shake the vial to mix the vaccine with the liquid before use.
SAQ 2.2 (tests Learning Outcomes 2.2 and 2.3)
Read Case Study 2.1 and then answer the question that follows it.
Case Study 2.1 Birke’s story
Birke is two months’ pregnant with her first baby. She is at the antenatal clinic and hears Nurse Ayele telling a group of women about neonatal tetanus, a disease that causes death in newborn babies. Nurse Ayele talks about the injection that women can get to protect themselves and their babies. Birke asks for the injection.
‘I am sorry,’ says Nurse Ayele, ‘I can’t give you tetanus toxoid now. It’s too early in your pregnancy and it might harm the baby.’
Birke is surprised. She says: ‘My friend told me that the health workers in Dembi Health Post give these injections to every woman the first time she goes to the antenatal care clinic, even if she is only one month pregnant. They say it is not dangerous.’
- Who is following the correct procedure: Nurse Ayele or the health workers in Dembi Health Post?
Answer
The health workers in Dembi Health Post are correct. TT vaccine should be given to all pregnant women during their first contact with a health facility, regardless of the gestational age of their pregnancy. So Nurse Ayele should give Birke the TT immunization.
SAQ 2.3 (tests Learning Outcomes 2.4 and 2.5)
Complete Table 2.9 by filling in the management of the adverse events listed.
| Adverse event | Managment |
|---|---|
| Low-grade fever | |
| Soreness at the injection site | |
| Abscess at the injection site | |
| Swollen lymph glands | |
| Severe allergic reaction (rash, breathing difficulty, rapid pulse, dizziness or fainting) | |
| Coma and/or convulsions |
Answer
The completed version of Table 2.9 appears below.
| Adverse event | Management |
|---|---|
| Low-grade fever | Paracetamol syrup, 5 ml as required, up to a maximum of four doses |
| Soreness at the injection site | Paracetamol (as above); warm compress applied to the affected area |
| Abscess at the injection site | Amoxicillin syrup orally three times daily and urgent referral to a health centre |
| Swollen lymph glands | Refer the child to a health centre |
| Severe allergic reaction (rash, breathing difficulty, rapid pulse, dizziness or fainting) | Do not give another dose of this vaccine; refer the child to a health centre immediately |
| Coma and/or convulsions | Do not give another dose of this vaccine; refer the child to a health centre immediately |
SAQ 2.4 (tests Learning Outcomes 2.2 and 2.4)
The mother of a 10-day-old infant visited your Health Post. She told you that her baby has a small sore on her right upper arm following a vaccination given immediately after the birth. What would you tell the mother?
Answer
You should tell the mother that the sore is a result of BCG vaccination, which will protect her baby from tuberculosis. Reassure her that this is a normal reaction to the vaccine and that the small sore is a good sign that the vaccine is working effectively. Tell her the sore will heal in about two weeks and leave a small scar, which is harmless.
SAQ 2.5 (tests Learning Outcomes 2.1 and 2.5)
What should you do if an infant is brought to you for routine EPI immunization at 14 weeks and you find that he or she has a temperature of 39oC? Is this a contraindication for vaccination?
Answer
This child has a high-grade fever. Give paracetamol syrup (5 ml) and refer the child to a health centre; do not vaccinate until the child recovers.