Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Sunday, 8 March 2026, 6:27 AM
Immunization Module: Immunization Safety
Study Session 7 Immunization Safety
Introduction
Immunization programmes will only be successful if immunization is practised safely. In this study session, you will first be reminded about how to keep vaccines safe and give injections safely (already taught earlier in this Module). You will also learn in more detail how to identify vaccine reactions and what to do if adverse events following immunization (AEFIs) occur. Finally, we will explain about methods of safe disposal for used injection equipment, discarded vaccines, vials and ampoules, and other waste such as used cotton swabs.
Learning Outcomes for Study Session 7
When you have studied this session, you should be able to:
- 7.1 Define and use correctly all of the key words printed in bold. (SAQs 7.1, 7.2, 7.3 and 7.5)
- 7.2 Explain how to keep vaccines safe and maintain their quality, including the correct practices for using multi-dose vials. (SAQs 7.2, 7.3 and 7.4)
- 7.3 Describe how to deliver safe injections in immunization sessions, how to treat mild vaccine reactions, and how to avoid adverse events due to programme errors in your immunization service. (SAQs 7.2 and 7.4)
- 7.4 Identify clients with contraindications to immunization and describe the appropriate actions to take if adverse events following immunization (AEFIs) occur. (SAQs 7.3 and 7.4)
- 7.5 Describe the equipment and methods used for safe waste disposal during and after immunization sessions. (SAQs 7.2 and 7.5)
7.1 Importance of immunization safety
Immunization practice that is not safe affects not only the individual receiving the vaccine, but can also affect you and others in the community. Immunization injections are safe when the correct and potent vaccine is properly administered with sterile equipment that is subsequently disposed of safely.
Immunization safety includes the following components, which will be described in detail in the following sections of this study session:
- vaccine quality and safety
- injection safety
- avoiding adverse events following immunization (AEFIs)
- safe waste disposal.
The organisation of immunization sessions, good communication with parents, other caregivers, and the community, and the collection and monitoring of immunization data, are also crucial requirements for a safe and effective immunization programme. You will learn about immunization programme management in Study Session 8, communication and advocacy on immunization in Study Session 9, and techniques for monitoring your immunization programme in Study Session 10.
7.2 Vaccine quality and safety
7.2.1 Vaccine quality
Using vaccines of high quality is very important in order to protect your community against vaccine-preventable diseases. Using poor quality vaccines can have an adverse effect on the individual, and is also likely to upset the community as a whole, for example if there is an outbreak of a disease that should have been prevented by your immunization programme (Figure 7.1).

The first check on the quality of vaccines supplied to health facilities in Ethiopia is made by a national body authorised for this purpose. Other checks follow on their condition at every stage in the transport to health facilities around the nation. However, once vaccines are in your care, you are responsible for maintaining their quality by storing them at an appropriate temperature until they are used. All vaccines are sensitive to heat, most cannot be frozen, and some are damaged by bright light; so it is crucially important to ensure that they are not exposed to heating or freezing, and are kept out of direct sunlight. Therefore, as you learned in Study Session 6, the cold chain should be maintained at all times, from the original manufacturer of the vaccine until the moment of administration to your clients.
7.2.2 Safety of the cold chain
If vaccines are spoiled by incorrect storage conditions, they will not be effective in preventing the associated disease, and they could also cause adverse reactions. This is also true for the diluents (ampoules of special liquid) used to reconstitute the freeze-dried (powder) BCG and measles vaccines before use. Each of these vaccines has its own specific diluent, which cannot be used for another vaccine. Diluents can be stored at room temperature if there is no room for them in the refrigerator, but before use they must be completely chilled to between +2oC and +8oC, so they reach the same temperature as the vaccine they are being mixed with.
Which vaccines and diluents available for routine use in the EPI in Ethiopia should not be frozen? What action should you take if freezing occurs?
Pentavalent vaccine, PCV10, TT and OPV should not be frozen. Also, the diluents for reconstituting BCG and measles vaccines should not be frozen. If any of these vaccines or diluents becomes frozen, they are no longer effective and should be discarded.
Which vaccines can be frozen (under exceptional circumstances) for temporary storage?
The only EPI vaccines in Ethiopia that can be frozen are the freeze-dried powder vaccines (BCG and measles) before reconstitution. Normally, all vaccines should be stored at between +2ºC and +8 ºC in the main (chilled) compartment of the vaccine refrigerator.
7.2.3 Multi-dose open vial policy
WHO Policy Statement, 2000, The use of opened multi-dose vials of vaccine in subsequent immunization sessions.
In order to reduce vaccine wastage, the Ethiopian Federal Ministry of Health (FMOH) and the World Health Organization (WHO, 2000) have developed guidelines on how to continue using vials of some types of vaccines once they have been opened, so they are not discarded unnecessarily at the end of the immunization session. These vaccines are supplied in multi-dose vials containing preservatives, so each vial can be used for many doses. Opened vials that are returned to the refrigerator must be labelled with the date they were opened, so you know when to discard them.
Opened vials of OPV and TT vaccines are the only EPI vaccines used in Ethiopia that can be used in subsequent immunization sessions within four weeks until the vaccines in the vials are fully used, provided that all five conditions in Box 7.1 are maintained. These conditions must be observed at every immunization session at the Health Post or at an outreach site.
Box 7.1 Conditions for using opened vials of multi-dose vaccines
Expiry dates are written in the European calendar (not the Ethiopian calendar).
- The expiry date has not been passed, i.e. the date after which the vaccine should not be used for immunization.
- The vaccines have been stored between +2oC and +8oC under appropriate cold chain conditions at all times.
- The vaccine vial has not been submerged in water (e.g. from leaking ice-packs in a vaccine carrier).
- The vaccine vial monitor (VVM), if attached, has not reached its discard point (look back at Figure 6.8 in Study Session 6).
- A new sterile needle and syringe and standard infection-control procedures have been followed to prevent contamination of vials when vaccine doses were withdrawn previously.
Standard procedures to reduce the risk of infection, contamination and injuries were taught in Study Session 4.
(Source: Adapted from WHO, 2004, Immunization in Practice, Module 3, The Cold Chain, p.16)
Why do you think it is important to prevent opened vials from being submerged in water?
The rubber membrane protecting the top of an opened vial has been pierced by needles whenever previous doses were withdrawn, so water could get into the vial if it becomes submerged.
If the conditions in Box 7.1 have been maintained, opened vials of OPV and TT vaccines may be returned to your refrigerator at a temperature between +2oC and +8oC after an immunization session. Put them in the ‘use first’ box to remind you that they should be used first (before unopened vials) the next time you give immunizations with these vaccines. (Look back at Figure 6.12 and note the position of the ‘use first’ box in the front-loaded refrigerator.)
Vaccines that should not be returned to the refrigerator after an immunization session are:
- opened vials of reconstituted BCG and measles vaccines
- opened vials of PCV10 vaccine (which does not contain a preservative).
These vaccines must be discarded 6 hours after reconstitution, or at the end of each immunization session — whichever comes first.
If a PCV10 vial is opened for one child and another is not immediately available to be vaccinated with the remaining dose in the two-dose vial, you should:
- write the time that the vial was opened on the vial so you can discard it after 6 hours if it has not been used
- ensure that the vial is kept cool in the foam pad of the vaccine carrier
- ensure that the vial is kept away from potential contamination.
Any unopened vials of vaccine – including unopened pentavalent vaccine, which is supplied in single-dose vials – can be returned to the refrigerator at the end of an immunization session, providing that the expiry date has not passed, storage under cold chain conditions has been maintained at all times, and the VVM has not reached the discard point.
However, if the VVM indicates that the vaccine has reached its discard point, then it should of course be thrown away and not used. Any vaccine which has passed its expiry date should also be discarded. You will learn how to do this safely later in this study session.
7.4 Injection safety
Safety in giving injections is essential for both the client and the provider. Unsafe injections can lead to the transmission of diseases such as HIV and hepatitis. Unless the waste materials of the injection process are disposed of safely, they may result in the spread of infection and cause injury. A safe injection is one that:
- does not harm the recipient
- does not expose the provider to any avoidable risk
- does not result in dangerous waste.
You learned in detail about the equipment for giving safe injections in Study Session 4. Here we will remind you of the main types that may be available at your Health Post:
- Disposable, sterile, single-use syringes and needles, which are used once only and then disposed of safely. They are commonly used for mixing freeze-dried vaccines (BCG and measles) with their diluents and should never be re-used.
- Auto-disable (AD) syringes, which are the preferred type of injection equipment for administering vaccines and should replace all other injection equipment if possible. These are used once and cannot be re-used, because the plunger of the syringe cannot be pulled back again once it has been pushed forward to inject the vaccine.
- Pre-filled, single-use syringes, which already contain a single dose of the vaccine, and are made by the manufacturer in such a way that they can only be used once.
All types of injection equipment must be disposed of safely after use, as described later in this study session. But first, we remind you of the circumstances in which immunizations should not be given.
7.4 Contraindication to immunization
The EPI policy recommends that health workers should use every opportunity to check whether eligible children have been immunized, and to immunize them if they have not received all scheduled doses of all the EPI vaccines at the correct age. However, as you already know from Study Sessions 2 and 3, sometimes a child may be temporarily or permanently unfit to receive a specific vaccine. This is called a contraindication to immunization.
Minor illnesses such as upper respiratory tract infections or diarrhoea, with low-grade fever below 38.5oC, are not contraindications for immunizing children with EPI vaccines. Infants with a moderate or severe fever (above 38.5ºC) should be considered as temporarily unfit for vaccination until their condition improves. A good rule to follow is that:
- if you are seeing a sick child at the Health Post and he or she is well enough to go home
- or you have no reason to refer a sick child that you have seen at home
- then the child is well enough to be immunized.
However, there are some absolute contraindications to immunization, which mean that a child should not be immunized (Box 7.2). You need to be aware of these conditions, because they may seriously affect a child if he or she is immunized.
The contraindications for individual vaccines were explained in Study Sessions 2 and 3 of this Module.
Box 7.2 Absolute contraindications to immunization with EPI vaccines
- Do not give another dose of pentavalent or PCV10 vaccine to a child who developed convulsions or a severe allergic reaction soon after, or within three days, of receiving the previous dose. Severe acute allergic reactions include generalized skin itching, skin rash, difficulty in breathing, swelling of the mouth and throat, and signs of shock (low blood pressure and rapid pulse rate); the symptoms quickly get worse (this is what ‘acute’ means).
- Do not give any doses of pentavalent PCV10 vaccine to a child with recurrent convulsions, or another active neurological disease of the central nervous system (brain and spinal cord).
- Do not give BCG or PCV10 vaccines to HIV-positive infants with AIDS, or symptoms of HIV infection including chronic lung infections, tuberculosis and persistent serious diarrhoea.
Note that HIV-positive asymptomatic infants (without symptoms) should receive all EPI vaccines at the earliest age possible, according to the nationally recommended EPI schedule.
Why do you think symptomatic HIV infection (with symptoms) is an absolute contraindication for BCG vaccination? Think back to what you learned about BCG vaccine in Study Session 2, and the effect of HIV on the immune system (Communicable Diseases Module, Part 3.)
BCG vaccine contains live-attenuated TB bacteria. The defence against infection in people with symptomatic HIV infection is very low, because HIV has damaged their immune system. If they are given BCG vaccine, they can develop tuberculosis.
7.5 Adverse events following immunization (AEFIs)
An adverse event following immunization (AEFI) is the term used to describe any adverse event that takes place after immunization, which may or may not be caused by the immunization. There are five categories of AEFIs defined in Table 7.1 below.
| Adverse events | Description |
|---|---|
| Vaccine reaction | Event caused or precipitated by the vaccine when given correctly, and due to the inherent properties of the vaccine |
| Programme error | Event caused by an error in vaccine preparation, handling or administration |
| Coincidental event | Event that happens after immunization but is NOT caused by the vaccine, i.e. it is a chance association |
| Injection reaction | Event caused by anxiety about, or pain from, the injection itself rather than the vaccine |
| Unknown cause | Event for which the cause cannot be determined despite thorough investigation |
7.5.1 Mild reactions to vaccines
As you already know from Study Sessions 2 and 3, most immunizations do not cause any serious health problems. Any vaccine reactions that do occur are usually mild and last only a day or two. They may include:
- swelling, soreness and redness at the injection site
- a low-grade fever, particularly after pentavalent, DPT or measles vaccination
- a slight rash, most often after measles vaccination
- some babies may display irritability (they are easily upset), or malaise (they seem low in energy and not interested in anything), particularly after pentavalent vaccination.
However, if scheduled doses of vaccines are not given because of mild reactions to a previous dose, this will lead to delayed immunization, or no immunization at all. If you allow this to happen, you will miss opportunities to protect children from vaccine-preventable diseases. Therefore, you should take every opportunity to immunize children, even if they have experienced a mild reaction previously.
Managing mild vaccine reactions
Advice on managing the common vaccine reactions should be given to parents, as well as instructions to return if there are more serious symptoms. This will help to reassure parents about immunization and prepare them for these common reactions.
Paracetamol is useful for the common minor reactions. It eases pain and reduces fever. A feverish child can be cooled with a tepid sponge or bath, and by wearing cool clothing. Extra fluids need to be given to feverish children. For a local reaction, a cold cloth applied to the site may ease the pain.
7.5.2 Serious vaccine reactions
On rare occasions, a reaction to a particular vaccine can be serious, even so serious as to be life-threatening. When serious problems follow an immunization, rumours are likely to circulate in the community that immunization is not safe, and children may then not be brought by parents for immunization. This will have a damaging effect on the spread of infectious diseases in the community.
Can you explain why a measles epidemic is likely to occur in a community if not enough children receive measles vaccine?
[You learnt about herd immunity in Study Session 1.] The reason is because the herd immunity in the population will be too low to prevent the measles viruses from spreading; it can pass from infected children to the many susceptible children who have not be immunized.
Table 7.2 summarises the possible serious adverse reactions to different vaccines that you may very rarely encounter. For example, acute flaccid paralysis following OPV occurs about once in every 1–10 million children vaccinated.
| Vaccine | Serious adverse event | Estimated period of onset (time after immunization) |
|---|---|---|
| BCG | Abscess (collection of pus) | 1–6 weeks |
| Swollen lymph nodes in the armpit | 2–6 months | |
| Bone disease | 1–12 months | |
| Pentavalent or DPT | Severe acute allergic reaction | 0–1 hour |
| Continuous screaming | 0–24 hours | |
| Brain disease, seizures (convulsions, fits) | 0–3 days | |
| Abscess | 1–6 weeks | |
| HepB | Severe acute allergic reaction | 0–1 hour |
| Paralysis | 1–6 weeks | |
| Measles | Severe acute allergic reaction | 0–1 hour |
| Abscess | 1–6 weeks | |
| OPV | Acute flaccid paralysis | 4–30 days |
| TT (women) | Severe acute allergic reaction | 0–1 hour |
| Nerve damage in the arm | 2–28 days |
However, most adverse events following immunization are not due to reactions caused by the vaccine. We will look at the other causes of AEFIs next.
7.5.3 Other causes of AEFIs
The most common causes of AEFIs are programme errors.
Programme errors
A programme error is the term given to an error caused by improper use of safety procedures or injection techniques. Common programme errors are summarized in Box 7.3.
Box 7.3 Incorrect immunization practices leading to possible AEFIs
- Failure to store vaccines correctly: inadequate maintenance of the cold chain at all times, leading to heat-damage or freezing of vaccines.
- Reconstitution errors: incorrect reconstitution of freeze-dried vaccines through use of the wrong diluent, the wrong amount of diluent, inadequate mixing of the vaccine powder with the diluent, or use of diluents at room temperature (they should be chilled to between +2ºC and +8ºC, the same temperature as the vaccine before mixing).
- Administration of vaccine by the wrong injection route: e.g. subcutaneous injection of BCG (it should be given intradermally), or subcutaneous or intradermal injection of pentavalent, PCV10 or TT vaccines (they should all be given intramuscularly).
- Administration of vaccine into the wrong site: e.g. giving intramuscular injections to infants in the buttocks, instead of in the outer thigh muscle; this error can lead to nerve damage.
- Administration of contaminated vaccine or diluent, or using unsterile injection equipment: this transmits infection and may cause a local abscess, or a more serious blood-borne infection such as HIV or hepatitis.
- Re-use of vaccines beyond their discard point or expiry date, or re-use of reconstituted vaccines or opened vials of PCV10 after more than 6 hours; these vaccines should be discarded and should never be returned to the refrigerator after an immunization session.
- Contraindications ignored, e.g. when a child who has had a severe reaction after a previous dose of a vaccine is immunized with the same vaccine, or a child with symptomatic HIV infection is given BCG or PCV10 vaccines.
Programme errors are mostly related to mistakes made by the health worker, which can be prevented through proper training. They may also be due to faulty equipment (e.g. a badly functioning refrigerator), or inadequate supplies of sterile injection equipment and other essential materials.
Coincidental events
An adverse event that follows an immunization may not have any association with the vaccine or the vaccination procedure – it may simply be due to coincidence. For example, a child may already be in the latent period of an infection, i.e. already infected but not yet showing any symptoms. When the symptoms appear a day or two after the immunization, the parents may conclude — incorrectly — that the vaccine has caused the infection. It is important that you investigate all AEFIs and explain to parents and the community why and how adverse events may follow an immunization simply as a chance effect.
Injection reactions
Sometimes the fear of being injected with a needle or the pain from the injection may cause a child to become very upset, perhaps even fainting or vomiting. This may also occur occasionally in women given TT vaccine. Take care to reassure the vaccinated person and any caregiver who is with them that the vaccine itself is harmless and their symptoms are due to anxiety, which will rapidly disappear.
AEFIs of unknown cause
Very rarely an adverse event occurs following immunization that cannot be attributed to any known cause, despite thorough investigation. You need to be alert to the possibility that unfounded rumours about a vaccine may start to circulate in the community. Be honest about the situation and explain that the cause is unknown but it is very unlikely to be due to the vaccine. You must report the AEFI so that the health authorities with responsibility for vaccine safety can record the event in case another similar event occurs following this vaccine, for example in another part of the country.
7.5.4 How to avoid AEFIs
In order to avoid AEFIs as much as possible, you should follow these guidelines:
- Fulfil the five requirements for using opened multi-dose vials (see Box 7.1).
- Do not vaccinate clients with absolute contraindications to immunization (see Box 7.2).
- Always reconstitute vaccines with the diluent supplied by the manufacturer. Never use another diluent.
- Discard reconstituted vaccines and PCV10 six hours after reconstitution, or at the end of the immunization session, whichever comes first.
- Do not keep other drugs in vaccine refrigerators if at all possible. If you have to keep other injectable drugs in the same refrigerator, put them on a separate shelf. AEFIs are likely to occur if you give an injection of a drug instead of the vaccine by mistake, or use a drug instead of the correct diluent.
- Prepare immunizations in a clean area where contact with blood and body fluids is unlikely.
- Use a sterile needle and syringe to prepare every injection dose immediately before administering it. Do not prepare several syringes in advance and do not re-use injection equipment.
- Do not touch the needle and never leave it in the top of the vaccine vial.
- If you use auto-disable (AD) syringes, AEFIs due to contamination should not occur.
- The child should be held firmly, so that there cannot be a sudden movement during the injection.

7.5.5 Detecting and responding to AEFIs
In your immunization activities, you should monitor, investigate, treat, refer and report the following AEFIs:
- all injection site abscesses
- all swelling in the armpit, particularly after BCG immunization
- all hospitalizations following immunization that occur within one month
- any other severe or unusual medical incident following immunization within one month
- all deaths that occur within one month of an immunization
- all medical events believed to be caused by immunization and about which people are concerned.
If you come across a suspected case of AEFI you should treat the affected person within your professional capacity, as described in Study Sessions 2 and 3, and refer him or her urgently to a higher health facility for further investigation and treatment. The patient should be accompanied by a responsible caregiver who has a clearly written referral note from you, explaining all relevant details.
If the AEFI is related to a known programme error, you must take immediate action to correct the cause. Liaise with the parents and community leaders to explain the cause of the AEFI, if it is known. You will learn more about communication in immunization programmes in Study Session 9.
A mother brings you an infant who has a large abscess on his arm. The abscess is at the site where you gave him an immunization against BCG ten days previously. What action should you take?
Keep the site of the abscess clean. Give amoxicillin syrup three times daily and refer the child urgently to a higher health facility.
All AEFIs, including those reported immediately during the month, should be counted in routine, written, monthly surveillance reports. (You will learn how to do this in Study Session 10.)
7.6 Safe waste disposal
The immunization programme should not put the community in any danger. Proper disposal of waste is an important issue and should always be planned from the very beginning. In particular, you need to plan how you will dispose of the used vials, ampoules, syringes and needles after an immunization session.
Disposal of medical waste is also taught in the Hygiene and Environmental Health Module, Part 2.
What other waste will need to be disposed of?
There will also be contaminated cotton swabs for cleaning the skin with alcohol or antiseptic before giving an injection, and pressed onto the injection site afterwards.
In this final section, you will learn about possible methods of disposing of waste safely. You may not have access to the best waste disposal method, so you may need to think about what innovations could be made to ensure that you keep the environment safe for the local community. You will need to select the most appropriate disposal method and site for the particular circumstances in your area.
7.6.1 Safety boxes
In order to avoid anyone being pricked by used needles and other ‘sharps’ (e.g. lancets), they should always be discarded with care. Immediately after injecting a vaccine, the syringe and needle should be placed in a nearby Safety Box (Figure 7.2).
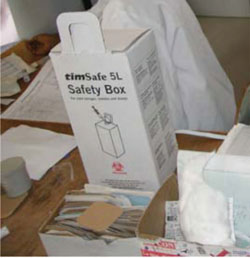
A five-litre safety box can hold about 100 needles and syringes. It is important not to wait until the safety box is completely full before disposing of it. It should be closed when it is about three-quarters full.
Can you explain why safety boxes should not be filled?
If the safety box is full, you could injure yourself on something sharp near the top of the box when you try to add more injection equipment.
7.6.2 Incineration and other methods of burning waste
Incineration means burning at very high temperatures under controlled conditions in an incinerator designed for this purpose. Incineration is a good way of disposing of waste, because it completely destroys needles, syringes, glass vials, and infectious agents by burning at very high temperatures. However, you may not have an incinerator (also known as a ‘protected hearth’) within reach of your Health Post. You could discuss the need for a proper facility for waste disposal with others in your community, such as Agricultural Extension Workers and kebele leaders. It might be possible to build an incinerator for you all to use (Figure 7.3).

Burning in a metal drum (container burning)
If you can get a suitable container such as a metallic drum with both ends removed (Figure 7.4), you can burn your medical waste in it. The drum should be placed in a fenced area that has been cleared for this purpose. Place four bricks on the ground, with spaces between them and a metal screen or grate on top. Place the open base of the drum on the metal screen and put another screen on top. The metal screens are to allow air to flow around the burning waste so the fire gets hotter, and to reduce the amount of ash flying out of the top. Put the safety boxes and some paper, dry leaves, or small sticks into the drum, sprinkle them with a small amount of kerosene (if available). Put paper under the drum, between the bricks, and set light to it so the flames rise through the metal screen.
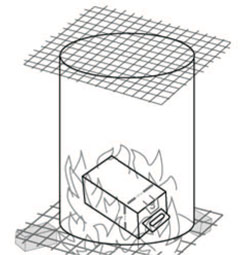
When the waste has completely burned and everything has cooled, put on heavy protective gloves and carefully scrape out any tiny pieces remaining in the bottom of the drum. Put them in a box and carry it to the Health Post waste pit for burial (see below).
Open pit burning
Open pit burning of waste is a common practice in many rural communities (Figure 7.5). The pit should be dug at least 2 metres deep in a fenced area. The waste should be placed in a closed and sealed box, such as a safety box, to be burnt. However, the problem with this method is that unburnt pieces may be blown by the wind and scattered around the pit, or the fire may go out and not destroy some of the waste. Ideally, you should watch while the waste is burning until you can see that everything in the box has been completely burnt. When the pit is three-quarters full of burnt waste, it should be covered with a deep layer of soil and (if possible) topped with concrete. It should be clearly marked so that everyone is aware that they must not dig up the contents.

7.6.3 Burying without burning
This method of disposing of medical waste involves digging a deep hole (known as a sharps pit) in a fenced area and burying the waste in the safety box, or another sealed container. If possible, the pit should be constructed with cement walls and a water-tight cover. It may be difficult to find a large enough space for repeated disposal of waste by this method. If the hole is not deep enough, the waste might become exposed when the top soil is washed away by rain or wind. Children or animals may dig up the waste unless the pit is protected by a secure fence. For these reasons, this method should be regarded as a last resort as the primary method of waste disposal. However, a pit should be constructed for burial of any small fragments remaining after waste has been burnt in an incinerator or metal container.
In the next study session we turn to other aspects of immunization programme management, in particular how you can increase the coverage rate in your catchment area.
Summary of Study Session 7
In Study Session 7, you have learned that:
- Safe immunization is essential in order to have a successful immunization programme.
- Immunization safety should include everyone involved: the client, the health worker and the community as a whole.
- You should follow standard infection-control procedures, and the guidelines on vaccine preparation and the re-use of open multi-dose vials, to ensure that vaccines and immunizations are safe and effective.
- Most vaccine reactions are mild and should not prevent the child from being immunized again. Serious adverse events following immunization (AEFIs) are rare, and are often due to programme errors by health workers. They may also be due to anxiety about the pain of an injection, or they may be coincidental events or due to unknown causes.
- Children with a minor illness (e.g. low-grade fever, respiratory infection, diarrhoea) should still be immunized according to the routine EPI schedule. Manage their symptoms and reassure their parents.
- Absolute contraindications to immunization are convulsions or a severe acute allergic reaction soon after a previous dose, or existing neurological disease. Infants with symptomatic HIV/AIDS should not be given BCG or PCV10 vaccines.
- Serious AEFIs should be reported immediately. All AEFIs should be reported in monthly written surveillance reports.
- Immunization waste must be disposed of safely to ensure that there is no danger to the community. If an incinerator or protected hearth is not available locally, safe waste disposal can be achieved by container burning or open pit burning in a protected area, or (if there is no other alternative) burial in a sharps pit protected by a fence.
Self-Assessment Questions (SAQs) for Study Session 7
Now that you have completed this study session, you can assess how well you have achieved its Learning Outcomes by answering the following questions. Write your answers in your Study Diary and discuss them with your Tutor at the next Study Support Meeting. You can check your answers with the Notes on the Self-Assessment Questions at the end of this Module.
SAQ 7.1 (tests Learning Outcomes 7.1 and 7.3)
- a.What is meant by a safe injection?
- b.Give three examples of possible programme errors in vaccine preparation or administration.
Answer
- a.A safe injection is one that does not harm the client, does not expose the provider to any avoidable risk and does not result in dangerous waste.
- b.Look back at Box 7.3 for descriptions of programme errors, i.e. due to incorrect immunization practices.
SAQ 7.2 (tests Learning Outcomes 7.1, 7.2, 7.3 and 7.5)
Which of the following actions could result in an infection being transmitted to your clients when you inject them with a vaccine? In each case, explain why or why not.
- A Allowing a freeze-sensitive vaccine to become colder than +1oC for a short time.
- B Allowing opened multi-dose vials of vaccine to become submerged in melted water in a vaccine carrier.
- C Using auto-disable syringes for every immunization.
- D Removing the cap from a disposable needle and holding it by the adaptor before you fit it onto the syringe.
- E Attempting to replace the cap on a used needle before you place it in the safety box.
Answer
The actions that could expose your clients to infection when you immunize them are:
- B Allowing opened multi-dose vials of vaccine to become submerged in melted water in a vaccine carrier. Contaminated water can infect the vaccine if it leaks into the vial through the tiny holes made by the needles used to withdraw vaccine previously.
- D Removing the cap from a disposable needle and holding it by the adaptor before you fit it onto the syringe. Infection could be transferred from your hands to the needle.
Actions A, C and D do not pose in infection risk to your clients, but you should know that:
- A Allowing a freeze-sensitive vaccine to become too cold will reduce its effectiveness.
- C Using auto-disable syringes for all immunizations is the best way to avoid any infection risk to your clients.
- E Attempting to replace the cap on a used needle poses an infection risk to you!
SAQ 7.3 (tests Learning Outcomes 7.1, 7.2 and 7.4)
Five minutes after you immunized a 6-week-old baby with her first dose of pentavalent vaccine, she became short of breath and developed a widespread rash. Her pulse became rapid and her blood pressure dropped.
- a.What is the name for this condition?
- b.What action should you take? (You will need to think back to earlier Modules in this curriculum to answer this part of the question fully.)
Answer
- a.These are the symptoms of a severe acute allergic reaction, with signs of shock (fast pulse and low blood pressure).
- b.You should refer the child immediately to the nearest health facility – this is a potentially life-threatening reaction to the vaccine. As you should know from earlier Modules, you should keep the baby warm at all times, tell the mother to continue breastfeeding if the child will suckle and go with them if you can. If you cannot go you should send a clearly written referral note listing all the relevant details.
SAQ 7.4 (tests Learning Outcomes 7.2, 7.3 and 7.4)
Which of the following children should not be given an immunization and why?
- a.A healthy-looking one-week-old baby girl who you know is probably HIV-positive.
- b.A 10-week-old boy who developed a low-grade fever soon after the first pentavalent immunization, which lasted about 24 hours.
- c.A 10-week-old boy who had a convulsion soon after the first pentavalent immunization.
Answer
- a.This HIV-positive infant is well, so she can receive the birth dose of BCG vaccine and all the routine EPI immunizations according to the normal schedule at the age of 6 weeks.
- b.Immunization with pentavalent vaccine can result in low-grade fever, which usually resolves within 24 hours. This is not a contraindication, so the child should be immunized with the second dose of pentavalent and the other routine EPI vaccines scheduled at 10 weeks.
- c.This child should not be given another pentavalent immunization. Convulsions soon after immunization are an absolute contraindication to further immunization with the same vaccine.
SAQ 7.5 (tests Learning Outcomes 7.1 and 7.5)
List one potential disadvantage of each of the following methods of disposal of a safety box containing used needles and syringes after an immunization session:
- a.An incinerator at a health centre.
- b.Burning in a metal container.
- c.Burning in an open pit.
- d.Burying without burning in a sharps pit.
Answer
The potential disadvantages of disposing of a safety box containing used needles and syringes after an immunization session, using one of the following methods, are:
- a.An incinerator at a health centre may be too far away to be a realistic method of regular waste disposal.
- b.Burning in a metal container will leave fragments of waste that must be scraped out of the drum and buried safely.
- c.Burning in an open pit may result in fragments being blown about by the wind and scattered around the pit, or not being completely burnt if the fire goes out too soon.
- d.Burying without burning in a sharps pit could result in waste being exposed by soil being washed away, or children or animals digging it up.