Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 26 February 2026, 10:01 PM
My Seaweed Looks Weird: An Introduction to Seaweed Parasites
Introduction
Like other living organisms, algae are affected by many pests and diseases which will result in abnormal morphological states or, more simply, weird-looking seaweeds. Though they remain vastly under-documented, parasites are increasingly regarded as key players in natural ecosystems, but also as one of the most serious economic and environmental threats to seaweed aquaculture.
You should see this course as guiding your way through the research emerging within this field. The core content should take around 5 hours to work through. The further reading at the end of each section is entirely optional but will help you to extend and onsolidate your understanding. You will have the opportunity to test your understanding and gain a badge to recognise your learning through taking two quizzes. Before you attempt the quizzes, you should look at the Course and badge information page.
1 Getting started
To start, watch the following video, which will give you a first look at pathogenic interaction. In the video, you will be introduced to Noreen Hiegle, who is a PhD candidate investigating the interaction between the pathogen Paraphysoderma sedebokorensis and the microalgae Haemmatococcus pluvialis. In the video she presents both organisms and illustrates the life cycle of the pathogen. Try to pick out the main stages and pay attention to the language used. There is a practice quiz about the video at the end of this section.
Transcript: Video 1 Illustration of a pathogenic life cycle.
This short video illustrates the complexity of pathogenic life cycles, and highlights the fundamental steps required for a successful pathogen: recognition of the host, infection, reproduction and dispersion. Later in the course (in section 3.4.2), you will see what this pathogen really looks like.
You will see in the next section that pests and pathogens of algae are highly diverse. So are their respective life cycles. This course does not aim to list all pathogens, but to illustrate some of them with respect to their ecological and economic importance. This course will generally deal with pathology concepts. We therefore strongly advise you to get comfortable with some important terminology. Here are some to start. Furthermore, as you go through the course you will see the terms are highlighted and you can click for an explanation or a quick reminder.
Introductory glossary
Biotroph: An organism whose exclusive, natural growth environment is in or on living host cells.
Disease: An abnormal physiological or developmental condition in a host due to the persistent action of a biotic (i.e. living organism) or abiotic (i.e. environmental) factor. Any condition of an organism that is economically detrimental.
Epiphytism (Epiphytes): The association of an organism with the surface of a host.
Facultative parasite: A facultative parasite is an organism that may resort to parasitic activity, but does not absolutely rely on any host for completion of its life cycle
Hemibiotroph: A parasite that requires living host cells during part of its life cycle.
Necrotroph: A pathogen that kills host cells in advance of its own growth, and obtains nutrients from the dead host cells.
Obligate parasite: An organism that can only live as a parasite in nature.
Parasite: An organism that obtains nutrients or other benefits from a living host. Note that a parasite may or may not cause disease.
Pathogen: An organism that can cause disease.
Pests: Anthropocentric term for all unwanted organisms interfering with the host (pathogens, parasites, epiphytes and grazers).
Saprobe (saprotroph): An organism that feeds on dead or decaying organic matter.
Symptoms: Visible or detectable characteristics of a disease.
Further reading
Most of the above terms are adapted from the following phytopathology glossaries:
It is worth browsing these resources to familiarise yourself with key terms.
Learn more, test your knowledge
If you take the time to read through the links below, you will see that algal parasites, as well as their ecological and economical relevance, are not – or only briefly – mentioned. This paucity of available information the motivation for creating these learning materials.
This course does not focus on aquaculture, but on algal parasites in natural and farmed environments.
For a more general description of seaweed aquaculture, you might be also interested by more general info on the basics and benefits of seaweed and microalgal farming: Seaweed farming on Wikipedia
You might be interested in this article, which focuses on the Japanese Nori large-scale cultivation.
You might like to read about the benefits typically associated with seaweed and microalgal cultivation.
The structure of the course
The rest of the course is divided into two main sections. The first section looks at the role of parasites in natural ecosystems, highlighting their importance within food webs and their wider environmental impact. The second section looks at parasites in seaweed aquaculture. It looks at the most widely researched and commercially important seaweeds and also touches on emerging areas highlighting significant gaps in our understanding of the dynamics within these systems. The language is necessarily technical, but will be familiar to people with a background in the biological sciences. We have tried not to assume too much prior knowledge of seaweeds or parasitology, and where possible we provide simplified summaries and explanations of key terms and interactions. For clarity and consistency, in this course we will mainly use the broader terms ‘diseases’ (irrespective of their biotic or abiotic origin) and ‘parasites’ (including pathogens, epiphytes and grazers.)
Seaweed (as you saw in the video and associated activities above) parasitology is a specialist and emerging area of research. This course will introduce you to this research, summarising and synthesising key studies. However, to appreciate this area you need to follow the links in the ‘Further reading’ boxes which are dotted about throughout the course.
The core material in this course should take you around two to three hours to work through. The ‘Further reading’ will take longer, and you may get hooked
Before you move on to Section 2, you should take the following short practice quiz, which is based on the video you watched in the previous section.
2 Pathogens in ecosystems: naturally a key player
In this section we aim to illustrate the ubiquity and natural occurrence of parasites as a grounding for the later section which looks specifically at algal pathology in aquaculture. You will start with looking at familiar terrestrial examples and then move on to look at aquatic examples on algal blooms, on parasites impact on food web and wider environmental processes. We have taken this approach as often when there is a spotlight on a disease it relates to its social or economic impact.
2.1 Parasites in ecological communities
You will now explore the key role parasites play in ecosystems.
2.1.1 Parasites everywhere!
Parasitic organisms exploit host resources for their development and reproduction. This lifestyle is successful, and it is widely recognised that there are more species of parasites than all non-parasite species combined. In aquatic ecosystems parasites can be found at each level of the food web, and in all taxonomic groups such as viruses, bacteria, fungi, oomycetes and so on. Figure 2 will give you a first glance of a few eukaryotic parasites, some of which will be described in more details later on in the course.
2.1.2 Parasites on parasites
Because every living organism is a potential shelter or food source, even parasites can be infected by other organisms. These are called hyperparasites. Hyperparasites are themselves prone to parasites, and numerous examples focused on land plants and animals can be found.
While algal pathology – as a scientific area – is only just taking off, records of aquatic hyperparasites affecting algal pathogens are still scarce and we will therefore use a land-based agriculture example as an illustration (see Figure 3).
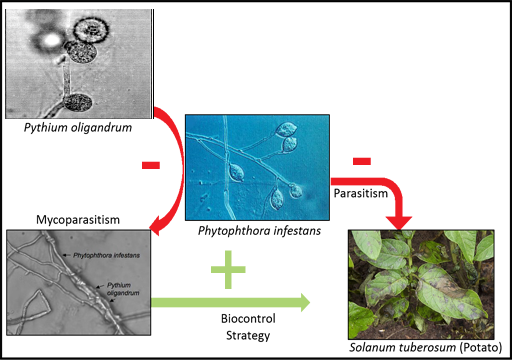
While P. infestans is the causal agent of the potato late blight (responsible for the Irish potato famine in the nineteenth century), P. oligandrum is a soil-borne organism that can colonise and decay P.infestans hyphae. Those naturally occurring relationships can be exploited for agronomic purposes: P.oligandrum is the main active ingredient of several biocontrol treatments used to protect cultures from a range of pests.
Will the development of algal pathology reveal such complex and promising interactions?
Further reading
Though under documented, hyperparasites can be also found in marine habitats. If you would like to learn more about how the development of algal pathology can reveal such complex interactions you could also read Gleason et al., 2014.
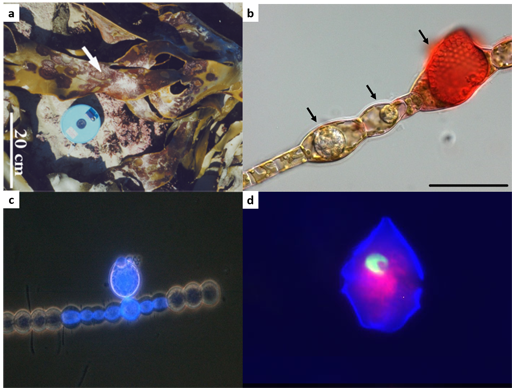
2.1.3 Impact on population dynamics: algal blooms
While obvious, it is worth stating that some parasites can ultimately kill their hosts and this can have a direct influence on host’s population dynamics in marine ecosystems. For example, certain dinoflagellate species of the genera Alexandrium and Cochlodinium produce toxins inducing paralytic shellfish poisoning and killing fish. They regularly form massive and harmful algal blooms commonly referred to as ‘red tides’ (Figure 4) and can cause tremendous public health issues and economic losses. Harmful algal blooms are an active research topic, where knowledge generated by scientists is integrated into policy statements and regulations.
Another big challenge is to understand the factors influencing red tides cycles, and parasites are increasingly recognised as key players in harmful algal bloom termination. The parasite Amoebophrya spp. can infect and kill a broad variety of dinoflagellates, including the above mentioned Alexandrium sp. and Cochlodiniumsp..
2.1.4 Infection of Cochlodinium polykrikoides by Amoebophrya
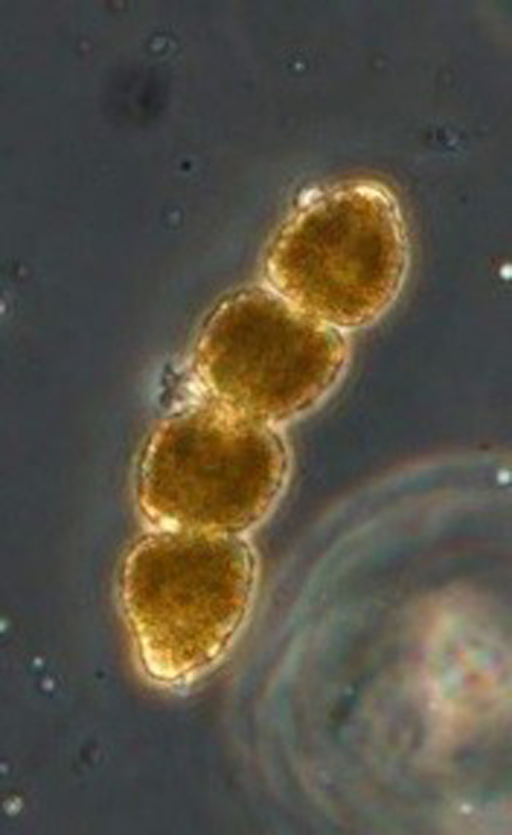
This alga typically grows as chains. It contains four cells on Figure 5. This alga is forming toxic blooms associated with fish kills.
Now, watch the video at the following link, which was recorded with an optical microscope and shows the final stage of infection: Amoebophrya spp. from the Bloom-forming Dinoflagellate Cochlodinium polykrikoides: Parasites not Nested in the “Amoebophrya ceratii Complex. Please note that this video has no sound. We have supplied a description of the content of the video if required.
The parasite Amoebophrya sp. alternates between a free-swimming infective stage (dinospore) and a growth phase (trophont) within its host. Following infection of Cochlodinium sp., the parasite develops intracellularly. The video showed the fully mature trophont leaving the infected Cochlodinium sp.. (a link to the corresponding article is given in the ‘Further reading’ box).
This trophont is short lived and will release new swimming dinospores that will infect new hosts. In blooming conditions (i.e. very high population density of the host algae), this exponential dissemination of the disease becomes highly significant. Velo-Suarez et al. (2013) studied the influence of the parasite Amoebophrya spp. on the termination of Alexandrium sp. blooms: they followed the abundance of Amoebophrya spp. through the full duration of an annual Alexandrium sp. bloom. During this bloom, high concentration of Alexandrium sp. is linked with an increase of infected Alexandrium sp., followed by a massive release of Amoebophrya spp. dinospores. On average, Amoebophrya spp. infected and killed around 30 per cent of the A. fundyense population per day in the end phase of the bloom.
Similar examples have been described in numerous fresh water and marine ecosystems illustrating the ability of parasites to influence dramatically population dynamics in aquatic ecosystems.
Further reading
If you want to explore further the content of this section, you might like to read the following articles which have more information images and videos illustrating these relationships:
Velo-Suárez et al., 2013. This article also details the life cycle of Amoebophrya on Alexandrium.
Kim and Park, 2014. This article focuses on the Amoebophrya/Cochlodinium interaction. The supporting information contains the video clip mentioned in this section.
Canter and Lund, 1948. This article looks at fluctuations in the numbers of Asterionella formosa hass. in relation to fungal epidemics.
2.2 Parasite, food webs and energy flows in aquatic ecosystems
The example just looked at is dramatic and, for many, increasingly familiar. It illustrates a broader point: that the influence of parasites on the population dynamics of their hosts is deeply embedded in ecosystems and directly impacts interspecific competition and the structure of food webs. A well-known land-based example is the introduction of the rinderpest virus in 1889: the disease killed the majority of the African Savannah grazing ungulates, thus starving and reducing the population of predatory carnivores. With no grazers left, the grass grew tall and increased the frequency of fire, reducing resources for tree-feeding species such as giraffes.
Similar situations have been documented for aquatic ecosystems, for which the influence of parasitism on food web structure and energy flow is vastly recognised. In all environments, and at all trophic levels, parasites and pathogens are increasingly being considered of equal importance with predators for ecosystem functioning, both in pristine and disturbed ecosystems.
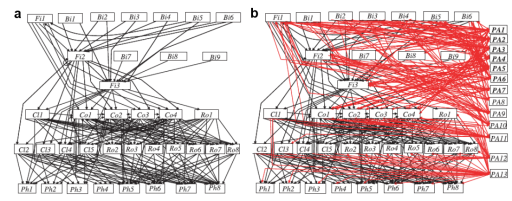
Figure 6 shows the complexity of ecosystem links. The thing to note is the significance of parasites: they typically account for the majority of total food web links and as such accelerate the recycling of organic matter.
2.2.1 Food web dynamics: the example of Chytrids
A concrete illustration of the role of parasites in shaping food web dynamics is the Chytrid ‘Mycoloop’ pathway developed by Kagami et al in 2007 and 2014 and is shown in Figure 7.
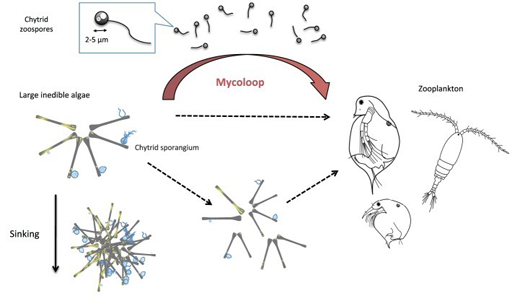
Parasitic chytrids are major pathogens of phytoplankton in aquatic ecosystems, and include a free-swimming zoosporic – the life-stage during which they actively search for new hosts. Chytrid zoospores are also subject to predation by grazing zooplankton because of their size, shape and nutritional quality. While large phytoplankton species such as Asterionella spp. are quite resistant to grazing, zooplankton can still eat Asterionella spp. chytrid parasite. The latter is therefore responsible for creating a food web link between zooplankton and phytoplankton. Additionally, as large phytoplankton colonies may also be fragmented as a result of chytrid infection, they become edible to zooplankton.
This mycoloop pathway may be particularly important to zooplankton when Asterionella spp. dominate the phytoplankton community.
A quantitative assessment of this food transfer was conducted by Rasconi et al., 2014 in two freshwater lakes. During blooms of ‘inedible’ algae (such as Asterionella spp.), 20 per cent of the energy created by primary producers was transferred to zooplankton through edible chytrid zoospores, with the zoospores representing roughly 60 per cent of the phytoplankton diet.
2.3 Environmental impact of algal pathogens – Coccolithophores and their viruses
The influence of parasites is not limited to flows of energy within systems, but can also play significant roles in broader environmental cycles. For example, Coccolithophores produce much of the ocean’s calcium carbonate, and are the major long-term source of atmospheric carbon dioxide. The influence of diseases on their population dynamics can therefore affect global geochemical processes. Coccolitophores like Emiliania huxleyi are one of the most abundant organisms present in the oceans. They can form blooms so enormous that they can be remotely sensed by satellites (this is shown by an image via Steve Groom and Plymouth Marine Labs in the Further reading below).
The rapid disappearance of these blooms has been a mystery for decades but there is now strong evidence that those bloom crashes are associated with viral infections and subsequent algal cell lysis. In a nutshell, as Coccolithophores have a major role in the Carbon cycles, their parasites also have important roles in global processes such as global warming.
Further reading
To explore the ideas in this section further, you might like to read the following:
- Hutchkins, 2011. This is a nature letter which summarises Coccolithophore carbon chemistry.
- A picture from Steve Groom and Plymouth Marine Labs which gives you a sense of how large these blooms can be. What looks like clouds on this satellite capture in in fact the reflected light from billions of coccoliths floating in the water column.
- A focus on one of E.huxleyi virus: Michaelson et al., 2010.
- Fuhrman, 1999.
- Suttle, 2007.
2.4 Summary of Section 2
Parasites are everywhere, are highly diverse, extend far way beyond the scope of human based activities and, as we have seen, algal parasites play a fundamental role in natural ecosystems. However, research and practical work on pathogens is always driven by human health, food or energy production and generally overlooked when it comes to organism interactions, specially algae.
Initially restricted to Asia, the potential of algal aquaculture sectors to meet diverse human needs is being recognised. This growing interest in algal diseases and their economic impact is likely an important driver for research. The next section will therefore focus on the main diseases and parasites that any algal aquaculture enthusiast is likely to encounter.
Further reading
If you are interested in exploring this area in more detail the following article considers some interesting questions around the role of parasites in ‘healthy ecosystems’:
3 Weird seaweeds in aquaculture facilities
As seaweed aquaculture develops in scale and scope researchers have found each algal species is plagued by different pathogens. This section of this course will give an overview of the diseases affecting the most important algal crops. It starts with the most economically important crops (i.e. red and brown seaweeds), and concludes with a look at developing sectors of green microalgae.
3.1 Red algae: Diseases in Pyropia Farms
The marine red alga Pyropia spp. (formerly named Porphyra), known as nori in Japanese and laver in English, is an important marine crop in Asia. It is used in many ways in the Asiatic cuisine, perhaps most famously as sushi wrap. It is the most valuable, and arguably one of the most advanced, of all algal industries, with a market value over $2 billion per year (see Figure 8).
Like any agricultural system, Pyropia (commonly called Nori) farms are plagued by many diseases. About 10 different diseases attack the nori plants including bacteria, viruses and fungal-like organisms. The two major diseases are caused by Oomycetes of the genera Pythium and Olpidiopsis. In Korea, where Pyropia cultivation has recently rapidly expanded new disease outbreaks are reported every year and can reduce the crop output by 20 per cent in certain areas, causing a general decrease of product quality and considerably lowering the market value of harvested Pyropia blades. It is also worth stating that global environmental shifts can stress and weaken algal crops, thus making them more prone to disease. The impact of those diseases is expected to increase with aquaculture expansion and global warming, and therefore have the potential to cause serious economic damages to sea farmers and related industries. The following examples are adapted from Kim et.al (2014). This paper provides useful and easy to follow insights for those wishing to extend their knowledge in this area.
3.1.1 Pyropia Red Rot disease
Pyropia spp. red rot is mainly caused by the oomycete Pythium porphyrae and is the most widely studied disease of Pyropia. Symptoms (shown in Figure 9) are characterised by small red patches on the blades in areas where the zoospores of P.porphyrae have germinated. Following germination, mycelium of P.porphyrae starts to colonise host cells intracellularly, killing them and progressively forming distinct patches of dead cells on the blades. Dead host cells turn from their natural reddish-brown colour to violet-red before they turn green and degenerate completely. The infection spreads quickly to other areas on the blade, and dead host tissue deteriorates forming numerous small holes. The holes merge into bigger holes, ultimately disintegrating the entire blade.
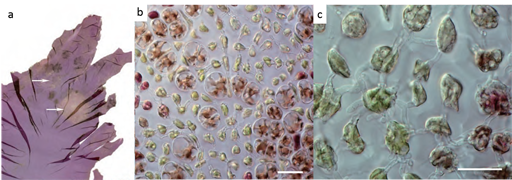
To date, Pythium porphyrae and Pythium chondricola have been reported as the main causing agents of the red rot of Pyropia. However, a recent paper identified a fungus of the genus Alternaria as another causal agent of the Red Rot disease.
Further reading
If you want to learn more about the recent discovery of Alternaria sp as a pathogen of Pyropia, you can read:
3.1.2 Olpidiopsis disease
The disease shown in Figure 10 has been reported in China, Korea and Japan. Initially thought to be a chytrid, Olpidiopsis is actually an obligate endoparasitic oomycete, infecting many different species of Pyropia. Symptoms (seen in Figure 10) are characterised by distinct bleached portions on blades at the initial stages and the development of greenish lesions upon spreading of the disease. The infection process starts when encysted zoospores of Olpidiopsis attach to the surface of Pyropia and produce thin germ tubes that penetrate the cell walls of the host. After entering the host cell, Olpidiopsis forms spherical multinucleate thalli, which develop into fully grown zoosporangia within the next two days. Zoospores escape through a discharge tube, which forms at the final developmental stage of the zoosporangium. Again, decay of tissue in infected areas quickly promotes the death of the whole plant.
3.2 Red algae: Carrageenan and Agar
Red algae are of considerable economic importance. The industry and research interests is not confined to Pyropia spp. to direct human consumption. Here we look at the Carrageenan and Agar industries
3.2.1 Kappaphycus – Carrageenan Industry
Carrageenans are polysaccharides extracted from certain kinds of algae whose gelling and emulsifying properties make them a widely used and valuable food additive. Kappaphycus alvarezii (see Figure 11) is one of the main carrageenan producing algae harvested in an industrial scale. It is a global industry with large scale Kappaphycus farms in the Philippines, Zanzibar, India, Fiji and Brazil. However, after a few years of favourable farming, producers encountered a number of diseases and epiphyte related issues.
Any changes in light intensity, ocean temperature and salinity cause stress to Kappaphycus, when stressed they produce organic compounds that attracts several types of bacteria, including those in the Vibrio-Aeromonas and Cytophaga-Flavobacteria complexes. Under those conditions, bacterial attacks will promote a characteristic softening, ‘bleaching’ or ‘whitening’ of the seaweed’s tissue called Ice-ice disease (Figure 12). Ice-ice disease can wipe out whole production sites. Outbreaks have occurred in almost every location where Kappaphycus production was introduced, with high ecological and societal impacts.
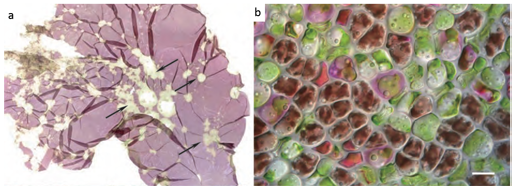
Epiphyte outbreaks also occur regularly in major carrageenophyte farms. They are mainly caused by red filamentous ceramiales (Vairappan, 2006; Vairappan et al., 2008). First signs of early epiphyte infection are observed with the appearance of black spots on Kappaphycus cuticle. Those spots are caused by embedded ceramiale sporelings that will emerge and mature in a matter of weeks. The multiple epiphyte infection sites will evolve further in a characteristic ‘goose-bump’-like symptom.
Epiphytic colonisation will promote productivity and quality decrease, bacterial secondary infections, and lead to farm collapse in the case of severe outbreaks.
3.2.2 Gracilaria – Agar Industry
Gracilaria is a red algae notable for its economic importance as a source of agar (more than 70 per cent of the raw material worldwide), bioactive compounds, as well as a food source for humans and various species of shellfish. Several species within the genus, including G.chilensis (a) and G.gracilis (b) are exploited among Asia, South America, Africa and Oceania, in wild or planted seaweed beds.
Similarly to Kappaphycus, Gracilaria farms are plagued by epiphytes that hamper productivity and decrease the market value of the crop. Overall the majority of those epiphytes also belong to the order Ceramiales, including Ceramium minuta, and Polysiphoniaforfex (Munoz et al., 2009)
Reports on diseases affecting Gracilaria beds are scarce and no terminology has been yet defined to describe specific symptoms (and there are no pictures available to date). Nevertheless, recurrent die-offs of Gracilaria gracilis in production facilities established positive correlations between disease symptoms (‘white-tip’ and ‘rotten-thallus’ syndromes) and the occurrence of epiphytic agarolytic bacteria (Schroeder et al., 2003). Among those, Pseudoalteromonas species are well represented, and were also associated with G. cordicata ‘Bleaching Stripe Disease’. A recent minireview of Egan et al., (2013) provides a list of confirmed and putative bacterial pathogens of macroalgae, some of which attacking Gracilaria.
In Hawaii, farmers and researches reported the Gracilaria Gall syndrome affecting G. tikvahiae. The disease slows or stops growth, reduces shelf-life and disfigures the seaweed, making it difficult to market. Diseased thalli display abnormal lesions or galls (small bump-like structures) along their stems or branches. Growth beyond the gall becomes twisted or contorted, and ‘witch’s-broom’-like structures may also appear at the ends of the branches. Research efforts were also focused on bacterial causative agents, but no further reports have been published since 1996.
3.3 Kelps – brown algae
The brown algae include many edible seaweeds, and a number of brown algae contains alginate that is extracted commercially and used in a large number of products. Cultivated kelp species including Saccharina spp. (formerly called Laminaria) and Undaria spp. are typically used for food while alginate are extracted from a wide variety of cheaper wild-harvested raw material.
The marine brown macroalga Saccharina latissima (sugar kelp) is the closest Atlantic relative of S. japonica, which dominates the Pacific Asian, particularly Chinese, seaweed industry. Although knowledge on the nature of Saccharina disease is scarce, the extensive Chinese farming reported many disease outbreaks. Table 1 indicates the most common diseases and their causes.
| Environmental diseases | Causes |
| Green rot disease | Poor illumination |
| White rot disease | Change in transparency and insufficient nutrients |
| Blister disease | Freshwater mixing with seawater after heavy rainfalls |
| Twisted blade disease | Excessive illumination |
| Pathogenic diseases | Causes |
| Malformation diseases | Hydrogen sulfide and sulfate reducing and saprophytic bacteria, e.g. Macrococcus |
| Sporeling detachment disease | Decomposing Pseudomonas bacteria |
| Twisted frond disease | Mycoplasm-like organisms |
There is now significant interest in the commercial exploitation of S. latissima aquaculture in several European countries. Already, disease outbreaks have been reported at S. latissima European seaweed cultivation facilities and wild kelp populations, the etiology is unclear. The gaps in our knowledge and the emerging nature of the research is illustrated by this video from Miriam Bernard on a windy shoreline. She is a PhD student studying the defence and resistance of S. latissima against endophytic pathogens.
Transcript: Video of Miriam Bernard
Putative pathogens are endophytic filamentous algae that could affect kelp growth, and less well studied fungi, bacteria and viruses. For example the brown algal endophytes of the genera Laminariocolax and Laminarionema could be highly prevalent in European wild kelp populations and are currently studied. They presumably invade the seaweed stipe and frond, and in some instances could severely perturb morphogenesis.
3.4 Emerging area of algae research: green algae …
We close by considering emerging aquaculture sectors, with Ulva spp. farming being developed as an alternative to wild harvesting, and culture of microalgae for high value products.
3.4.1 Macroalgae: Ulva lactuca brown spots
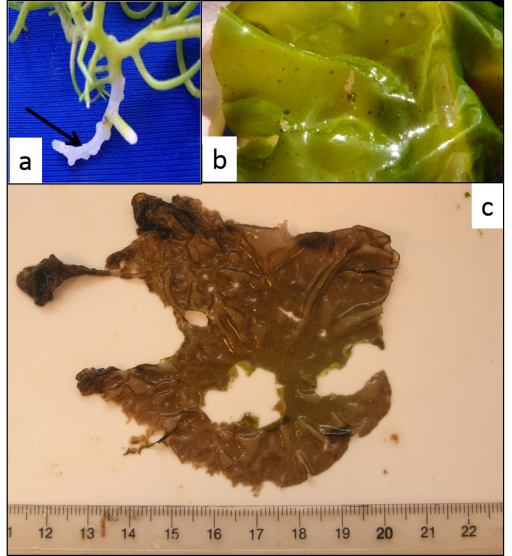
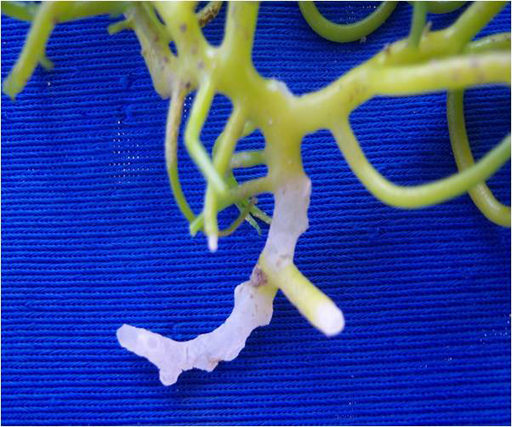
Ulva (1) is the main green macroalgae used for human food. It is harvested worldwide and is being cultured in many parts of the world in pilot commercial systems. Research into wild populations has found the epipthytic brown algal genus Myrionema (2,3,4) to be widely present in Ulva wild populations. It causes Brown spots (2) and blades can be highly infested. The reduction of growth rates due to nutrient competition, and a decreased market value of the final product are a concern for commercial producers.
3.4.2 Microalgae
Haematococcus spp.
Haematococcus pluvialis Flotow is a chlorophyceae freshwater microalga increasingly attracting interest for industrial mass-scale cultivation. Under stressful conditions, for instance, nitrogen depletion, this species produces high levels of the ketocarotenoid astaxanthin (a pigment commonly used in cosmetics, nutraceuticals and as animal feed).
H. pluvialis represents an important natural source of this compound. There is established research into Green algal pathogens (e.g. Chlorella virus) within natural systems. However, mass cultivation has accelerated the rate of disease and pathogens are becoming critical. Among those, Hoffman et al. (2008) first reported Paraphysoderma sedebokerense as a new pathogen of H.pluvialis in Israel and established its taxonomic position in the Blastocladiomycota closely related to the plant pathogen genus Physoderma. Since then, patent applications relating to its control have been developed in the USA and China, strongly suggesting that P. sedebokerense causes widespread production losses in industrial facilities across the world.
Further reading
Like many algal parasites, the life-cycle of P.s is highly complex. Our lab has discovered previously uncharacterised stage that you will find in the following article:
3.4.3 Scenedesmus dimorphus
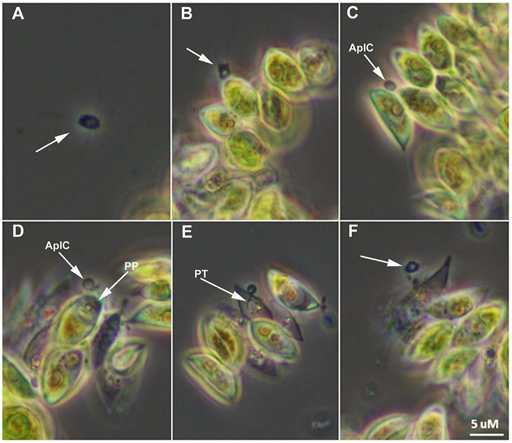
The production of biofuels using algae is an attractive technology that could mitigate the ongoing depletion of fossil reserves and their impact onimpacts/influence on climate change. Open pond mass cultivation of the green alga Scenedesmus dimorphus is considered as one of the most promising strategy for cost effective production of algae. Again, with the recent implementation of those culture strategies, multiple reports of parasitic attacks that can destroy mass cultures in a matter of days have been reported raising concern for this developing sector. Amoeboaphelidium protococcarum belong to the Cryptomycota clade and has been recently described in a study by Letcher et al, 2014. Its life cycle is illustrated in Figure 16.
In the figure, A. Motile aplanospore (arrow) is proximal to host. B. Aplanospore (arrow) encounters Scenedesmus dimorphus cells and attaches. C.
Aplanospore has completed attachment and has formed an epibiotic cyst (AplC). D.
Parasite protoplasm (PP) has been injected into the host cell and clearly visible within the cell. E. Clear view of the parasite penetration tube (PT); progeny begin to mature within parasite sporangium. F. Dehiscence of aplanospore cyst occurs and progeny (arrow) are released from the infected cell.
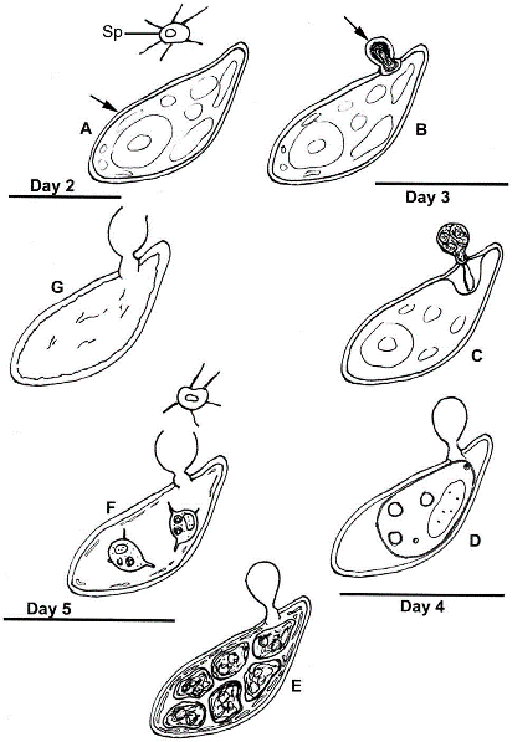
At Day 2: A. Abundant healthy algal cells (arrow) and abundant amoeboid, filose pseudopodiate aplanospores (Sp) are evident. B. A minority of algal cells indicate infection by the presence of a cyst (arrow) attached via an appressorium to the algal cell wall.
Day 3: C. The walled, stalked aplanospore cyst has enlarged, a penetration tube penetrates the host cell wall, and a host reaction at the site of infection is evident. D. In some algal cells a membrane-bound, developing parasite protoplasm occupies a portion of the interior of the host cell, and a host/parasite interface is evident; the empty remnant of the aplanospore cyst persists.
Day 4: E. Cleaved aplanospores within the host cell are surrounded by fused and fragmented plasma membrane; the empty remnant of the aplanospore cyst persists.
Day 5: F. A minority of host cells retain one or a few unreleased aplanospores, a sub-apical portion of the empty remnant of the aplanospore cyst has dissolved, and abundant aplanospores occur outside host cells. G. Most infected host cells are empty, but retain the remnant of the aplanospore cyst.
Further reading
If you want to learn more about the biology of Aphelids and the ongoing work concerning their classification, you could read the following articles:
3.5 Summary of Section 3
This section gave you a quick tour of the most commonly found interesting and engaging examples that you might encounter as a seaweed enthusiast or as seaweed practitioner. It provided you with an overview the important crops and their pests, and critically it highlights the emergent nature of knowledge within this area. As seaweed aquaculture becomes more important and a global industry the importance of these parasites within natural and farmed systems increases, as does our knowledge, including a better understanding of what we do not know. We hope you find the gaps as stimulating as what is known: we do!
Conclusion
Since the early 1950s, exponential growth of algal aquaculture in Asia has led to the global production roughly doubling in volume every decade. After having been mainly restricted to Asia, the economic potential of algae as a food, energy and high value product source is now being universally acknowledged, giving rise to numerous pilot large-scale cultivation projects worldwide.
The most serious economic and environmental concern that immediately arises with the introduction of new farming practices, including cultivated seaweeds, is the loss of crops to pests. A global, proactive research agenda on pests is urgently needed to secure the sustainability and future success of the seaweed industry. While diseases of algae have been known for a long time, there is a consensus that the recent shift towards intensive algal production methods correlates with more damaging outbreaks.
During this course, you have learned that pests of algae can be found in highly diverse taxa, from viruses, bacteria, other algae, to fungi and oomycetes. Because pathogens are ubiquitous actors of pristine ecosystems, they are bound to be one of the major challenge in algal cultivation. As more and more diseases are discovered with every new cultivated species, the worldwide highly dynamic growth of algae aquaculture will surely stimulate research on the diversity of pathogens, their role in shaping aquatic ecosystems, and how to deal with our weird seaweeds.
As stated before, algal diseases are still vastly under documented, and chances of encountering new parasites are high. If you encounter one of the parasites described in this section, or an unreported organism, let us know!
End-of-course quiz
To finish this course and obtain your badge, you should now carry out the end-of-course quiz. You might find it useful to open the quiz in a separate tab or window so you can easily refer back to the course as your work your way through the quiz.
Get involved
SAMS and the GlobalSeaweed Network is actively collaborating with the ‘Capturing our Coast’ project to integrate seaweed pathology in the free training of Citizen Scientists. More information can be found at the Capturing our Coast website.
We have set up a space on the site ‘Capturing our Coast’ to allow you to share ‘your data’.
You can also contact us directly:
References
Acknowledgements
This free course was written by Dr Yacine Badis and Dr Claire Gachon in the frame of the GlobalSeaweed project thanks to the NERC IOF Pump-priming + scheme (NE/L013223/1).
Many thanks to Ronald Macintyre for insightful comments, constructive criticism and constant assistance in the writing process and design of the learning journey.
Other contributors include Critical Friends (Rafael Loueiro and Andrea Garvetto) who carefully revised final course draft, and Noreen Hiegle and Myriam Bernard for sharing their PhD presentations.
Some pictures in this course were kindly provided by:
Pr Inga Kjersti Sjøtun - healthy Ulva picture, and
David Fenwick – Gracilaria gracilis picture
Pr W.J Fry – Phyophthora infestans micrograph of sporangia
Dr Pieter Van West – Pythium oligandrum mycoparasitism picture
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this free course:
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.
Images
Figure 1 © SAMS/Yacine Badis
Figure 2 © Claire Gachon
Figure 3 From: Hani M. A. Abdelzaher, M. A. Shoulkamy and M. M. Yase (2004) Kinds, Abundance and Pathogenicity of Pythium Species Isolated from Maize; © Yacine Badis; © William James Fry; Rasbak (2011) Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. A copy of the license is included in the section entitled GNU Free Documentation License. https://commons.wikimedia.org/ wiki/ File:Phytophthora_infestans_potato_%27Dor%C3%A9%27,_aardappelziekte_Dor%C3%A9.jpg
Figure 4 Released into the Public Domain, August 2005. P. Alejandro Díaz and Ginny Velasquez, https://commons.wikimedia.org/ wiki/ File:La-Jolla-Red-Tide.780.jpg
Figure 5 Smithsonian Institute.
Figure 6 © Claire Gachon
Figure 7 Kagami, M., Miki, T. and Takimoto, G. (2014) Mycoloop: chytrids in aquatic food webs. Front. Microbiol. 5:166. doi: 10.3389/fmicb.2014.00166 Copyright © 2014 Kagami, Miki and Takimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
Figures 8, 9 and 10 Kim et.al (2014). Note this is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure 11 CC BY 2.0, https://en.wikipedia.org/ wiki/ Kappaphycus_alvarezii#/ media/ File:Kappaphycus_alvarezii.jpg
Figure 12 CC SA 3.0 Ronald Simbajonhttps://commons.wikimedia.org/ wiki/ File:Ice-ice_disease_on_Kappaphycus.jpg
Figure 13 © Yacine Badis; CC BY-NC (Attribution-Non-Commercial) Hommersand, Max H. (2007); Permission received by David Fenwick
Figure 14 © Yacine Badis. Adapted with permission of Inga Kjersti Sjøtun Kjersti.Sjotun@uib.no. © SAMS Martina Strittmatter
Figure 15 © SAMS Martina Strittmatter
Figures 16 and 17 Letcher, P.M., Lopez, S., Schmieder, R., Lee, P.A., Behnke, C. (2013) ‘Characterization of Amoeboaphelidium protococcarum, an Algal Parasite New to the Cryptomycota Isolated from an Outdoor Algal Pond Used for the Production of Biofuel’, PLoS ONE, vol. 8, no. 2, doi:10.1371/journal.pone.0056232. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Videos
Video 1 © SAMS
Video 2 © SAMS
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.