Pain and aspirin
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Friday, 10 May 2024, 5:48 PM
Pain and aspirin
Introduction
In this course you will find out that the sensation of pain is caused by the release of a chemical called prostaglandin that stimulates the nerve endings and sends an electrical message to the brain. Inhibiting the formation of prostaglandin reduces pain and we will see, by looking at the specific shape of the molecules involved, how aspirin can so inhibit the formation of prostaglandin. To make the most of the material of this course you will need to use an organic molecular modelling kit such as the one that is supplied by Molymod™ to Open University students who study the module that this course comes from.
This OpenLearn course provides a sample of level 1 study in Science.
Learning outcomes
After studying this course, you should be able to:
demonstrate general knowledge and understanding of some of the basic facts, concepts and principles relating to the development of medicines
demonstrate knowledge and understanding of the science behind the development of some drugs to achieve particular tasks
demonstrate knowledge and understanding of how chemical bonding determines the properties of compounds and provides an explanation for the mode of action of drugs
apply this knowledge and understanding to address familiar and unfamiliar situations
express unit concepts in an objective and factually correct way.
1 Why does it hurt?
The relief or avoidance of pain must be one of the major driving forces behind medical research. In this course we start the discussion about relief of pain.
When we experience the sensation of pain it is likely that something is happening that the brain needs to know about, so it can direct us to whatever damage-limiting action is needed. We hurt because we have genes that constructed a body able to feel pain.
Without such a mechanism it is likely that life would be much shorter, with less opportunity to pass on our genetic code. It seems, therefore, that being able to feel pain is a state of affairs favourable to the continuing success of the species. This is unlikely to be foremost in the thoughts of someone who has just broken their leg or poured boiling water over their foot, but the fact remains that feeling pain is part of the defences that enable us to stay alive (Figure 1).
2 How does it hurt?
This is a useful question because once we know the mechanism of pain sensation we can do something about alleviating it.
When tissue is injured there follows a rapid release of ‘messenger’ chemicals that stimulate the nerve endings. Electrical impulses are relayed through the nerves to the spinal column and to the brain, which registers the sensation of pain. It usually, but not always, also directs our attention to the site where the damaged tissue initiated the pain message.
Drugs to alleviate pain act to interrupt this flow of information. There are three basic types grouped together by the way in which they work.
Drugs such as aspirin act at the site of the injury to stop or at least reduce the production of messenger chemicals that stimulate the nerve endings.
Another class of drugs, the opiates such as codeine and morphine, act on the central nervous system (brain and spinal cord). Sometimes, the aspirin-like and codeine/morphine-like drugs are combined into one remedy, for example co-codamol and co-proxamol.
Local anaesthetics.
For the time being, we will concentrate on aspirin, to illustrate the development of drugs and how they are able to achieve their effects.
3 The aspirin story
As long ago as 400 BC the physician Hippocrates, from the island of Kos (now a popular Greek holiday destination) prescribed a concoction made from willow leaves to help relieve the pain of childbirth. Ever since then (and probably even before) herbal remedies based on the leaves or bark of willow trees have been used for the alleviation of pain and fever. In the 1840s the chemists of the day were able to extract the substance salicin from the bark of willow trees by treating it with boiling water. They isolated and identified salicin and found that it was the active ingredient in the pharmacological action of willow bark. This, then, became the lead compound from which other pain-reducing drugs have been developed, including aspirin, of which millions of tablets are taken annually throughout the world.
In 1870 van Nencki, working at the University of Basle, showed that in the body salicin is converted to salicylic acid. As natural products such as salicin usually only occur in small amounts and are often difficult to synthesise, a common strategy is to find a related compound that can easily be made in large amounts in the laboratory. In this case, salicylic acid looked promising, but treating patients with salicylic acid instead of salicin, whilst affording the same reduction in symptoms, also had the marked disadvantage of causing severe irritation to the mouth, the gullet and the stomach lining – an early example of undesirable side-effects. So this modification of the lead compound salicin was not an improvement. (Salicylic acid can be used to remove warts from the skin, e.g. the preparation sold as ‘Compound W®’ – a solution of salicylic acid in acetic acid. Its ability to destroy tissue is then of use!). Note that the symbol ® means that the name is a registered trade mark.
A further modification was to convert the salicylic acid into sodium salicylate and see what effect this had. This resulted in a compound that still had the same pharmacological properties as salicylic acid but had the advantage of reduced irritation. A new disadvantage appeared, though – it tasted awful!
The German chemist, Felix Hofmann, then set about modifying the structure of salicylic acid. He made one small change at a time and tested each new product on his father who had rheumatism, until in 1898 he arrived at a product that was as effective as salicylic acid, but lacked the severe irritation and taste problems. This new material was given the name aspirin and, after further clinical trials was manufactured and marketed by the company Bayer, as the first medicinal drug to be available in tablet form.
4 The molecules involved
4.1 Salicylic acid
The structural formula of salicylic acid, 2.1, looks quite complicated. However, it becomes less daunting if you unpack it a bit. One of the first things to do when confronted with an unfamiliar structure is to check that all the valencies are correct (four for carbon, two for oxygen and one for hydrogen). If any atoms have the wrong valency, it follows that there is a mistake somewhere and the molecule does not exist as drawn. It looks OK for the structure of salicylic acid. You probably noticed that some of the carbon atoms have two bonds joining them to another atom. These are called double bonds and they contain four electrons, two per bond. They are quite common in chemical structures. There are two types of double bond in salicylic acid, carbon-carbon double bonds (C=C) and a carbon–oxygen double bond (C=O). Two of the thinner, more flexible bonds in the model kit are used when making models requiring a double bond in the structure. Make a model of each type of double bond in 2.1 and keep them for using later.
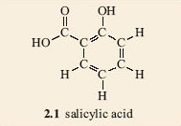
Let's have a closer look at the salicylic acid molecule. For a start, focus on the ring part of the structure. If the two groups attached to the ring (the side chains) are removed and replaced with hydrogen atoms, we are left with the hydrocarbon, benzene. This is a liquid, present in coal-tar, which used to be widely used as a solvent by chemists until it was discovered just how poisonous it is. Nowadays benzene is a product of the petrochemical industry.
Activity 1
1. Make a model of a benzene molecule, 2.2, with your model kit.
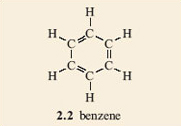
What do you notice about the model that makes it different to all the previous molecular models that you have made?
Answer
All the atoms lie in one plane; the ring structure is flat (planar). The presence of the double bonds is the cause of this.
2. Now turn your attention to the rest of the salicylic acid molecule. Remove the hydrogen from one of the benzene's carbons (it does not matter which one) and replace it with an oxygen joined to a hydrogen (an OH group). When this type of reaction is carried out for real, chemists refer to it as a substitution reaction. The structure that you have made is 2.3. You may need to rotate your model, or the —OH group on it, to match 2.3.
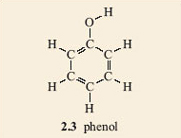
Note that the C—O—H sequence in 2.3 is not linear. Also the —O—H group in 2.3 can rotate freely around the C—O bond, giving many possible overall shapes for the molecule only one of which is planar.
The presence of the —OH group gives the molecule 2.3 particular properties that are not possessed by benzene. Such a group is called a functional group. The —OH functional group is called a phenol (pronounced fee-nol) group if it is joined to a benzene ring. The same word, phenol, is also used as the name of the compound you made (2.3) consisting of a benzene ring carrying an —OH group and no other substituent groups.
Activity 2
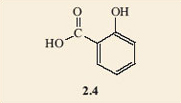
Now look at the other group on the benzene ring in salicylic acid, 2.1. Make a model of this group and substitute it for another hydrogen atom on your model of phenol. This time you will have to be a bit more particular about which hydrogen atom you substitute. It must be on a carbon atom that is adjacent to the phenol group. There is still some choice, though, as there are two of them.
Does it matter which of the two carbon atoms you change?
Answer
It appears that perhaps it does, as it looks as if there are two possible isomers. These are 2.4 and 2.5, and would certainly represent different molecules as they are not superimposable on each other. The difference is that 2.4 has a single bond between the substituted carbons and 2.5 has a double bond between these two carbon atoms. Notice how the structural formulae have been simplified. The benzene ring part of the molecule can just be shown as a hexagon with alternate double and single bonds. Chemists recognise that there is a carbon atom at each of the corners and each carbon atom must carry another group or atom to keep its valency correct. When no group is shown on a corner carbon atom, it is understood that a hydrogen atom is bonded to it. This style stops the drawn structures from getting too cluttered.
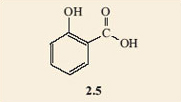
It is found that only one product results when the hydrogen atoms on two adjacent carbon atoms in a benzene ring are substituted by any two functional groups. All attempts to make two isomers end up producing the same product. This is an example of failure of the model to depict what the structures really are. In fact neither of structures 2.4 and 2.5 accurately depicts the structure of salicylic acid. A better model is a combination of both of them; you could regard it as a sort of average of the two structures, with one of the bonds in each double bond spread around the ring. This is often shown by drawing a circle in the ring, instead of three double bonds. The circle represents six electrons (two from each of the double bonds) shared round the ring. Structure 2.6 shows the structure of benzene drawn in this style and 2.7 depicts salicylic acid drawn in the same style.
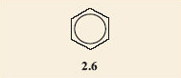

Because the ball-and-stick models are unable to show these ‘spread-around-the-ring’ electrons, the structures for benzene rings where the double bonds are shown separately will be used. However, where necessary it should be remembered that these double bonds are shared round the ring. With this in mind, 2.4 and 2.5 turn out to be representations of the same molecule, within the limitations of what the ball-and-stick models can show.
‘Spread-around-the-ring’ or ‘delocalised’ electrons do confer some special properties to benzene and similar compounds. Benzene is said to be ‘aromatic’ or to have ‘aromatic properties’. Although the word aromatic originated because many of these compounds had a characteristic odour it no longer means anything to do with smell – in fact some aromatic compounds have awful smells. It now just means a ring structure with delocalised electrons.
Note that the —COOH group that you have put on the benzene ring contains an —OH group. However, the close proximity of the C=O part of the molecule changes the properties of the —OH group. In fact the whole of the group (one carbon, two oxygens and a hydrogen atom) is named as one functional group. It is a carboxylic acid group and has a whole set of chemical properties that make it different to the —OH group. To simplify writing this functional group it is often abbreviated to —COOH or —CO2H. Some people refer to it as a ‘coo’ (rhymes with blue) group in conversation.
4.2 The functional group approach
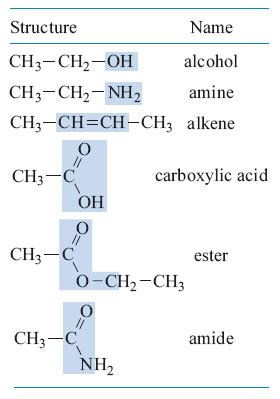
It is the classification of functional groups that simplifies the study of organic chemistry (the chemistry of compounds that contain carbon). With many millions of known organic compounds, and more being added by the day, it would be hopeless if their properties could not be systematised in some way. It turns out that a given functional group usually has the same chemical properties whatever carbon chain it is bonded to, so once the general properties of each functional group are known, all that is left to deal with are the exceptions. It is nearly always the functional groups that undergo change when an organic chemical takes part in a reaction. Fortunately there are relatively few functional groups, some of which are shown in Table 1. For convenience, and to keep the valencies correct, these are shown with a hydrocarbon chain attached. It is the shaded bit of each molecule that is the functional group.
Note that functional groups are parts of molecules but they do not exist in isolation. Molecules of compounds like CH3CH2OH (Table 1) contain the alcohol functional group, —OH, but they also include a hydrocarbon chain, CH3— CH2—. The compound is in the family of compounds called alcohols, all of which have an —OH group attached to a hydrocarbon chain that is often depicted simply by the symbol R.
4.3 Aspirin
Question 1
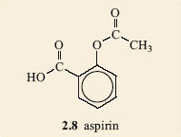
Compare the structure of aspirin, 2.8, with that of salicylic acid, 2.7. What similarities and differences can you see?
Answer
The structures look quite similar. They both have a benzene ring carrying two groups, on adjacent carbon atoms. In both of them one of the groups is a carboxylic acid group. But, salicylic acid carries a phenol group whilst aspirin does not.
Question 2
Can you identify the group that is carried by aspirin in the corresponding place to the phenol group in the molecule of salicylic acid? Have a look at options in Table 1.
Answer
You should have concluded that this is an ester group. If you did not identify this group correctly, try making a model. Remember there is free rotation about the single bonds and this should enable you to make it look like the ester group in Table 1.
You are going to study an important reaction between functional groups on molecules in this section. If you are new to chemistry you may not have seen chemical equations before, so before moving on work through Box 1 which provides you with a brief introduction to this topic.
Box 1 An introduction to chemical equations
When chemists want to refer to a chemical reaction in which bonds are broken and new bonds are formed to produce new molecules (the products) from other molecules (the reactants), they often do so by means of a chemical equation. The reactants are shown on the left and the products are on the right,
reactants = products
As atoms cannot be created or destroyed in chemical reactions, the total number of atoms of each element involved must be the same on each side of the equation, if the equation is to ‘balance’ correctly. The two sides are then linked by an equals (=) sign and the reaction is referred to as a ‘balanced reaction’.
For example, methane – the first molecule you made a model of and a fossil fuel gas – burns in the oxygen of the air to form carbon dioxide (CO2) and water (H2O). The reaction can be represented by the equation:
CH4 + 2O2 = CO2 + 2H2O
Count the atoms of each element on the left of the reaction and compare with the numbers on the right. They should be the same! Remember 2O2 means 2 × 2 = 4 atoms of oxygen (O).
Note the need for two molecules of oxygen and two molecules of water to balance the equation. The information that this equation contains is that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
Sometimes the numbers of the molecules are unimportant and we just want to focus on the formulae of the reactants and products, not how much of each is involved. Chemists often show this type of relationship with a reaction having an arrow (→) instead of an equals sign. The following reaction is in this style:
CH4 + O2![]() H2O
H2O
5 Some chemistry involving esters
Esters are produced by the reaction of a carboxylic acid with an alcohol and result from the formation of a new bond (Reaction 2.1). For example, ethyl butanoate, the major constituent of artificial pineapple flavouring, is made from the reaction of butanoic acid with ethanol.
![]()
There is a certain logic to the naming of these compounds. Note the endings on the names: -ic for the carboxylic acid, -ol for the alcohol and -ate for the ester.
Because the other product is water, this type of reaction is known as a condensation reaction. (There are other condensation reactions that do not involve the formation of water, but they do involve two molecules joining together to form a larger molecule with the elimination of a different smaller molecule, e.g. ammonia or hydrogen chloride, instead of water. We will not be concerned with these other condensation reactions.) This condensation reaction is common to nearly all carboxylic acids, R1COOH, and alcohols, R2OH. So, we can write the general reaction, 2.2, where the abbreviation R1 represents the rest of the carboxylic acid molecule and R2 represents the rest of the alcohol molecule. Check through Reactions 2.1 and 2.2 and make sure you can follow the way in which some of the atoms move from one molecule to another as the reaction takes place. We have colour coded the reacting groups in Reactions 2.1 and 2.2 to help you see this change.

The use of the symbol R to represent the rest of the molecule is common in organic chemistry. It enables us to focus on the parts of the molecules that matter (the functional groups) without the formulae being cluttered up with the parts that are not reacting.
Question 3
Which carboxylic acid and alcohol would you use to make isopentyl acetate, 2.9, a constituent of banana oil? (Hint: you need to identify R1 and R2.)

Answer
Reaction 2.3 shows the required carboxylic acid and alcohol.
![]()
An important thing about this reaction is that, although it shows an ester forming from a carboxylic acid and an alcohol, it could equally well have been written the other way round. It would then show the reaction of an ester with water, to produce a carboxylic acid and an alcohol. This is an example of a chemical reaction that can run in either direction, rather unsurprisingly known as a reversible reaction. They are very common and sometimes you see the use of the symbol ![]() instead of =. The reverse of the condensation reaction above is known as a hydrolysis reaction, reflecting the fact that it is reaction with water. The word is derived from hydro (water) and lysis (splitting), so hydrolysis is literally splitting with water, which accounts for the fact that any hydrolysis reaction gives two products.
instead of =. The reverse of the condensation reaction above is known as a hydrolysis reaction, reflecting the fact that it is reaction with water. The word is derived from hydro (water) and lysis (splitting), so hydrolysis is literally splitting with water, which accounts for the fact that any hydrolysis reaction gives two products.
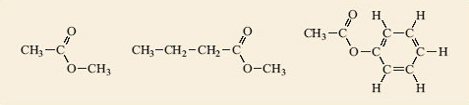
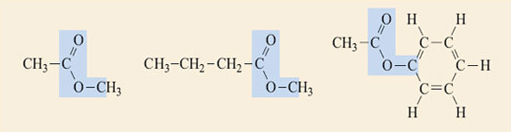
Question 4
Identify the ester groups in the compounds shown in Figure 2. Write down the reactions that produce each of the esters and thus deduce the structures of the carboxylic acids and alcohols needed to make them.
Answer
In Figure 3 the ester groups are highlighted in blue.
Reaction 2.4 shows the generalised equation for the formation of esters (in a slightly different form from Reaction 2.2).
![]()
Reactions 2.5 to 2.7 show how each ester would be formed using this reaction
![]()
![]()
![]()
Now look back to the structures of salicylic acid, 2.7, and aspirin, 2.8.
Question 5
See if you can pick out the carboxylic acid that is involved in converting the phenol group in salicylic acid into the ester group in aspirin.
Answer
You should have decided it was CH3—COOH, acetic acid, but may well have written its formula in a different style or shape. This does not matter, it is still acetic acid so long as the atoms are joined together in the same order as in our structure,
Activity 3
Complete your study of the relationship between salicylic acid, acetic acid and aspirin by making models of salicylic acid, 2.7, and acetic acid. Place them side by side so that the phenol group (—OH) of the salicylic acid is next to the —COOH of the acetic acid. Then remove the H atom from the phenol and the —OH from the acetic acid and join the two fragments together. You have made an aspirin molecule, 2.8. What else have you made?
The other compound that you have made is water, H2O (remember this is a condensation reaction), provided you joined the unwanted H atom to the —OH. The complete reaction is shown in Figure 4.
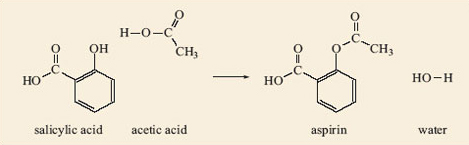
You should be able to see how the reaction in Figure 4 can take place in the reverse direction (hydrolysis), with the water molecule splitting the aspirin molecule into molecules of salicylic acid and acetic acid. If you have an old bottle of aspirin tablets you may find they have a slight smell of vinegar, especially if they have become damp. As you have seen, when aspirin reacts with water the carboxylic acid that it forms, by hydrolysis of the ester group, is acetic acid. This is the compound responsible for the smell of vinegar.
6 How does aspirin relieve pain?
Aspirin acts at the site of damaged tissue to block the start of the nerve signal to the brain, the mechanism by which we experience pain (Section 2). It does this by inhibiting the formation of prostaglandin which is the active agent responsible for the sensitisation of the nerve endings. Prostaglandins (there are several related types) are responsible for a lot of physiological events in addition to the start of the pain signal at the nerve ending. For example, they are responsible for inflammation, fever and the clotting of platelets in the blood, so it is easy to see why aspirin is such a useful drug. However, a prostaglandin also increases the formation of protective mucus in the gut, so aspirin-induced suppression of its formation can cause irritation and bleeding.
Before an explanation of aspirin's activity at the molecular level can be developed some more chemical ideas are needed. Prostaglandins are synthesised in the body's cells from arachidonic acid, 2.10. This is a carboxylic acid containing a chain of 20 carbon atoms in its molecule. 2.10 shows this structure.
![]()
Although this structure looks rather complicated at first, it is relatively simple in that it has an unbranched carbon chain terminating in a carboxylic acid group. It does have the added complication of four carbon–carbon double bonds but the real influence of these is not apparent until one looks at the shape of the molecule. We have made a model of 2.10 and photographed it, but before we look at that you need to return to your model kit and have a look at the shape surrounding the atoms joined by a carbon-carbon double bond.
Activity 4
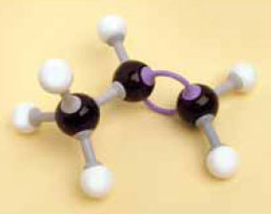
1. Make a model of the compound ethene, C2H4, using the appropriate number of bonds and keeping the valencies correct for all the atoms.
You should have found that you needed a double bond between the two carbon atoms (remember to use two of the longer bonds in the kit for this) and should have ended up with the structure shown in Figure 5.
If you think back to previous models you made, you should recall ethane, CH3—CH3, the second member of the homologous series known as the alkanes. Note the similarity in the names. The ending –ane has been changed to –ene, but the rest of the name (eth-) is preserved because it refers to the fact that there are two carbon atoms in the molecule. Ethene is the simplest member of the homologous series known as the alkenes. The simplest alkane, methane, does not have a counterpart in the alkene series, as it is not possible to have a double bond if there is only one carbon atom in the molecule. After this, though, the alkenes follow a similar naming sequence as the alkanes, so you have propene, butene and so on.
2. Now take your model of ethene. Remove one of the hydrogen atoms, extend the carbon chain by one carbon atom and add the appropriate number of hydrogen atoms to keep all the valencies correct. You now have a model of a propene molecule.
This should have been straightforward enough and our model is depicted in Figure 6.
3. Now take your model of propene and extend it by one carbon atom plus the appropriate number of hydrogen atoms to make a molecule of C4H8.
You probably found this less straightforward as you could join the extra carbon atom to any one of the three carbon atoms in propene and it looks as if you end up with several different molecules.
The molecules are shown in Figure 7. All these molecules exist and we need a way of naming them that makes it clear which molecule is being discussed.
The structure shown in Figure 7d does not present a problem. It has a methyl group attached to an alkene with three carbon atoms in the chain and so it is named methylpropene.
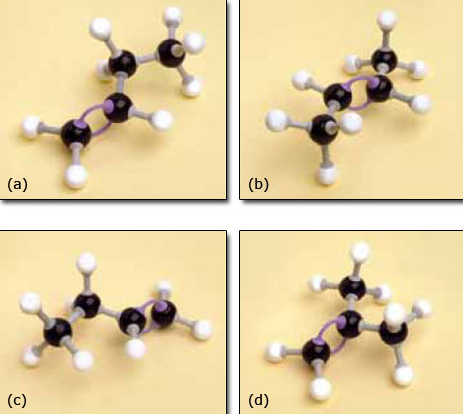
Figure 7
Before attempting to name the remaining structures, Figure 7a–c, we need a way to identify exactly which carbon atoms are joined to the double bond. This is done by numbering the chain, starting at one end, as shown in Figure 8.
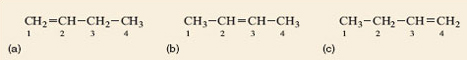
Figure 8
As Figure 7a and b cannot be exactly superimposed on each other they are molecules of different compounds. They are named but-1-ene and but-2-ene, respectively, the number in the name designating the lowest numbered carbon atom in the chain to which the double bond is joined. It might be expected that Figure 7c is but-3-ene but this is not the case because it is identical to but-1-ene (Figure 7a). It can be picked up, turned over and superimposed on but-1-ene, so is the same – try it with your models. By convention the lowest possible number(s) is always used in a name whenever there is more than one option. Because but-1-ene, but-2-ene and methylpropene have the same molecular formula (C4H8) but different structural formulae (showing which atoms are joined to which) they are isomers (Section 1.4).
You might have found that but-2-ene created a problem in that you needed to decide which side of the double bond to place the two —CH3 groups and the two hydrogen atoms. Our models are shown in Figure 9.
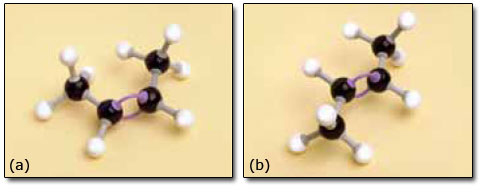
Figure 9
The problem was caused by an important feature of the model that is also possessed by the molecule itself. Two carbon atoms joined by a double bond cannot rotate relative to each other. This part of the molecule is therefore much more rigid than parts built with just single bonds. Cis-but-2-ene and trans-but-2-ene have the same structural formula and the same sequence of atoms is present in both. However, their geometry differs so one cannot be superimposed on the other, so they really are different compounds. They are known as geometrical isomers, or cis/trans isomers (Latin: cis, on this side; trans, across).
Returning to the structural formula of arachidonic acid, introduced earlier, the implications soon become apparent. The double bonds control the shape of the molecule.
![]()
There are four double bonds in the molecule, each of which can be cis or trans, so generating a pair of geometrical isomers. If all the different geometrical isomers of 2.10 are modelled that makes a total of 16 different possibilities in all! However, because double bonds prevent rotation within the molecule only one of the 16 isomers has the atoms in the correct place to form prostaglandin. The relevant isomer is the one where all the double bonds are cis, as shown in Figure 10. This isomer needs to react with oxygen and form the ring near the middle of the carbon chain (Figure 11). If any of the double bonds are trans, the atoms are too far apart to react to form the five-membered ring and the structure shown in Figure 11 could not form.
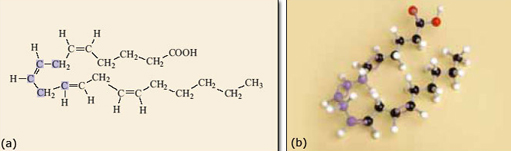
Figure 10
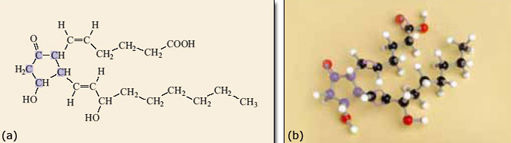
Figure 11
The formation of a five-membered ring in the arachidonic acid molecule to produce prostaglandin could not take place in the body without the action of enzyme molecules. These are compounds that increase the rates of reactions in living systems.
7 Enzymes
7.1 Enzymes: nature's catalysts
It will probably come as no surprise to you that chemical reactions, including the conversion of arachidonic acid into prostaglandin, do not occur instantaneously and the rate at which they take place can be very variable. Some reactions are over in a flash, such as the burning of gunpowder, and others take months, such as rust formation on a car. All chemical reactions can be speeded up by increasing the temperature of the reactants. In the laboratory one often ‘cooks’ reaction mixtures for, say, 30 minutes at 100 °C to make them react at a useful speed. However, the human body does not have this facility and has found alternative ways of speeding up the many chemical reactions that it needs to carry out.
Nearly all of the chemical transformations that go on in the body are helped along by enzymes. Enzymes are nature's catalysts, enabling otherwise slow reactions to occur rapidly. Enzymes are very effective at their job, enabling large numbers of reactions that would otherwise require quite extreme conditions, to be carried out in the body. Like all catalysts, enzymes are not used up or changed by the reactions they are speeding up. They are also highly specific in their action so many different enzymes are required to cope with all the different chemical reactions needed to keep our bodies working. For example, enzymes known as lipases assist with the digestion of fats by increasing the rate of hydrolysis of the ester groups within the fat molecules. The enzyme cyclooxygenase (COX) catalyses the formation of prostaglandin from arachidonic acid.
7.2 How enzymes work
Enzyme molecules have an ‘active site’ that is a specific shape for a given enzyme. It is here that reactant molecules are converted into products. The active site binds to and holds the reactant molecule in exactly the right position for the reaction to take place. Effectively it fits around the molecule rather like a glove fits around a hand. This very precise three-dimensional structure can only be achieved by enzymes being large complex molecules.
Because the enzyme fits the reactant like a glove, the active site will accommodate only a very small range of different molecules. The enzyme is said to be highly specific in its action. The relationship between enzyme and reactant has been likened to a lock and key (Figure 12); the enzyme is like a lock where only one type of key, the correct reactant, will fit the active site. Thus only a very specifically shaped molecule will interact with the enzyme and undergo a reaction. In Figure 12, although the three possible reactant molecules all have very similar shapes, only one can fit into the active site.
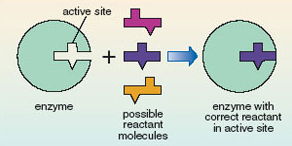
Figure 12
There are usually several steps in a chemical reaction – the reactants do not just disappear and turn into products without passing through a number of intermediate stages. This sequence of intermediate reactions is called the reaction mechanism. Catalysis occurs by the enzyme being intimately involved in the mechanism. Figure 13 shows a schematic representation of the stages that are involved. Enzymes provide an alternative, faster, reaction mechanism. In early stages of the mechanism the enzyme reacts with the reactant to give intermediates; however, the enzyme is regenerated in a later stage. Thus, the catalyst, an enzyme, is not consumed; it is continually recycled so one molecule of enzyme can catalyse the conversion of many reactant molecules into product molecules. Only a small amount of the catalyst is required. Since the catalyst is not consumed, it does not end up as part of the product and the overall reaction product is the same irrespective of whether it is catalysed or not; the catalyst merely speeds up the reaction.
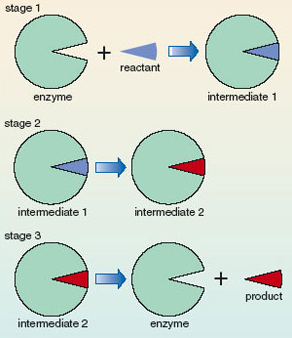
Figure 13
The way that enzymes work is an area of great interest to chemists, because if we know how enzymes control the conversion of reactants into products then it gives the chemist the opportunity to help out when things go wrong.
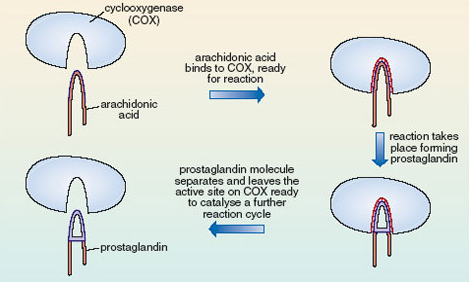
7.3 Formation of prostaglandin
Figure 14 models the way that the enzyme cyclooxygenase (COX) catalyses the formation of prostaglandin from arachidonic acid. Note how important the shape of the arachidonic acid molecule is. It needs to be just right to match the shape of the active site on the COX molecule and, as you have seen, of the 16 possible geometrical isomers, only one will fit. The one with all the double bonds cis has all the atoms in the right place to fit into the cavity containing the active site of COX and is the right shape to cyclise and form prostaglandin.
8 Enter aspirin!
Aspirin is able to release part of its ester group (Figure 15) in a hydrolysis reaction. Look again at the structure of aspirin, 2.8, and identify this group on the molecule. It is known as an acetyl group and accounts for aspirin also being called acetylsalicylic acid. The acetyl group on aspirin is fairly easily removed and can be available for forming another ester with an —OH group on another molecule; in this case, part of the structure that makes up the inside of the cavity in COX (Figure 16). The wiggly line in this reaction represents the rest of the COX molecule.
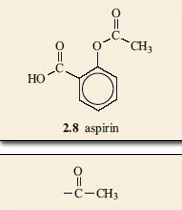
Figure 15
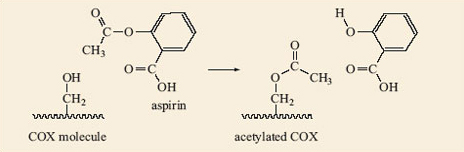
Figure 16
The —OH group in the COX cavity has become acetylated, i.e. had an acetyl group added to it. Quite simply this just makes the cavity of the active site of COX smaller. The arachidonic acid is no longer able to enter the cavity, so prostaglandin does not form, so the pain is relieved (Figure 17). This acetylation involves the formation of a covalent bond which is strong, so the acetyl group is not readily released and aspirin continues to relieve pain for quite a long time after the dose is taken.
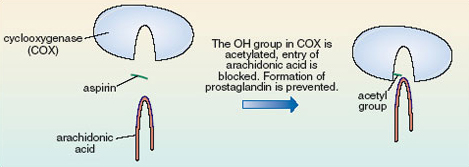
Figure 17
Question 6
Write a list of the main events in ‘the story of aspirin’, from the time of Hippocrates to when Bayer started marketing aspirin.
Answer
The steps in the development of aspirin are shown below. There are likely to be other perfectly acceptable answers that show slight variations.
| Step 1 | Willow bark and leaves known to be effective painkillers for thousands of years. Salicin extracted from willow bark during the 1840s. |
| Step 2 | Salicin (lead compound) tested and found to be the active ingredient in willow bark and leaves. |
| Step 3 | Structure of salicin investigated. |
| Step 4 | Salicylic acid synthesised. |
| Step 5 | Salicylic acid found to be effective but irritant. |
| Step 6 | Sodium salicylate made. Effective for pain relief, not irritant but tasted awful. |
| Step 7 | Synthesis of other compounds related to salicylic acid. |
| Step 8 | Hofmann tested them (on his father). |
| Step 9 | Aspirin emerged as the suitable compound. |
Question 7
What is the molecular formula of aspirin?
Answer
The molecular formula just tells you how many atoms of each type there are in one molecule of the compound, so for aspirin it is C9H8O4.
(There are six C atoms in the ring plus three in the side-chains, giving nine overall. There are four H atoms attached to the ring carbons that do not carry side-chains plus four H atoms in the side-chains, so a total of eight. There are two O atoms in each side-chain, making four in all.)
Question 8
Make a model of a molecule of cyclohexane, a hydrocarbon with molecular formula C6H12 that contains a six-membered ring of carbon atoms. Compare it to a model of benzene and comment on their different shapes.
Answer
You should have found that all the atoms in the benzene molecule lie in one plane, but this is not so with cyclohexane. This is because cyclohexane does not have any double bonds to restrict the shape that the molecule can adopt.
Question 9
Each cell in the human body accommodates around 2000 different enzymes but only a small quantity of each one. Why are there so many and why is only a small quantity of each required?
Answer
Enzymes are highly specific in their action. That is to say each enzyme will only catalyse a very small range of chemical reactions, or perhaps only one. As a human cell needs to carry out a very great number of different reactions it needs a wide range of different enzymes. It only needs a small amount of each enzyme because enzymes are not used up or converted into different chemicals when they perform their function of catalysing cell reactions, so are recycled.
Conclusion
In this course you have found out that:
The sensation of pain is caused by the release of a chemical (prostaglandin) that stimulates the nerve endings and sends an electrical message to the brain.
Pain can be reduced if the formation of prostaglandin can be inhibited.
Prostaglandin is formed, from arachidonic acid, in a cavity in the active site of the enzyme cyclooxygenase (COX).
Geometrical isomerism can be important in controlling the shape of molecules.
The specific shape of the arachidonic acid molecule is caused by four carbon–carbon double bonds in its carbon chain which limit the rotation allowed within the molecule. Only one of the isomers, in which all four double bonds are cis, has the atoms in the correct place for prostaglandin formation.
Aspirin can release an acetyl group, which bonds to the active site of COX and prevents arachidonic acid from entering the cavity, so inhibiting the formation of prostaglandin.
Acknowledgements
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence
Course image: Sage Ross in Flickr made available under Creative Commons Attribution-ShareAlike 2.0 Licence.
All other materials included in this course are derived from content originated at the Open University.
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
This free course is adapted from a former Open University course called 'Molecules, medicines and drugs (SK185)'.
Copyright © 2016 The Open University