1.4.1 Inhibitors of cell wall synthesis
As you saw in Activity 3, the cell wall is essential for normal functioning of the bacterial cell. Antibiotic inhibitors of cell wall synthesis block the production of peptidoglycan, the main component of the cell wall. Cross-linking between peptidoglycan chains forms a strong, mesh-like structure that gives the cell wall structure and rigidity (Figure 7) and protects the underlying cell membrane from osmotic damage when water moving into the cell by osmosis could cause it to burst, or lyse. Osmosis is where water moves through the membrane to try and balance the concentration of water molecules both inside and outside the cell.
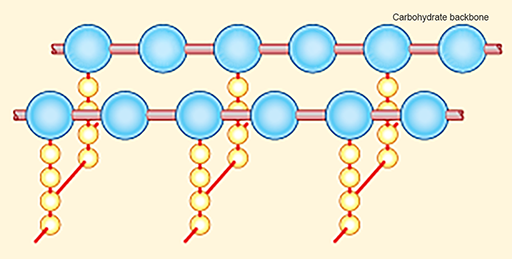
Figure 7 Structure and arrangement of peptidoglycan chains in the bacterial cell wall. Peptidoglycan molecules consist of a backbone of carbohydrate units with sets of amino-acid residues attached (yellow). They are cross-linked by bridges (red), providing structure and strength.
Show description|Hide descriptionThe diagram shows two adjacent chains of alternating N-acetylglucosamine and N-acetylmuramic acid units, represented here as large blue balls. Extending downwards from each N-acetylmuramic acid unit is a chain of four amino acid residues, shown here as small yellow balls. The ends of the amino acid chains on adjacent carbohydrate units are joined by glycine cross-bridges, shown here as red bars.
Disruption of the peptidoglycan layer of the cell wall can therefore result in cell lysis (Figure 8).
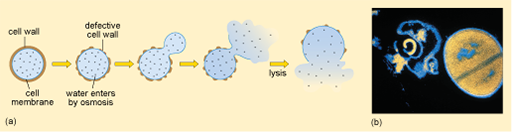
Figure 8 Lysis of a bacterium with a defective cell wall. (a) Diagram showing the sequence of events that lead to lysis. (b) Light micrograph of S. aureus: a lysed cell on the left and an intact dividing cell on the right.
Show description|Hide descriptionPart (a) is a schematic diagram showing the sequence of events that lead to the osmotic lysis of a bacterium. Initially the cell wall and the cell membrane beneath it are intact. As water enters by osmosis, the cell wall becomes defective. Eventually the cell contents and surrounding membrane expand through the defective cell wall, the membrane then ruptures and the cell contents spill out; that is, the cell lyses. In the light micrograph in part (b), the intact near-spherical cell appears orange–yellow on the black background; while the lysed cell has collapsed and lost most of its contents and so has a shrivelled shape and appears mostly black.
Examples of cell wall synthesis inhibitors are the β-lactam antibiotics. These include penicillin and its derivatives, and the cephalosporins. All β-lactam antibiotics contain a core chemical structure called a β-lactam ring (Figure 9) that determines the mode of action of this class of antibiotics.
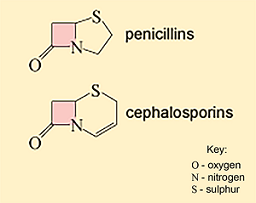
Figure 9 Core ring structures of two types of β-lactam antibiotics. The β-lactam ring is shaded pink in each case.
Show description|Hide descriptionThis figure shows the core ring structures of penicillins (top) and cephalosporins (bottom). They both contain a ring that is joined to the common beta-lactam ring (shaded in pink), but the structure of this ring differs between the two classes: the penicillins have a sulphur (S)-containing 5-membered ring with no double bonds; the cephalosporins have a sulphur (S)-containing 6-membered ring with one double bond.
The β-lactam antibiotics interfere with the formation of the peptidoglycan cross-links by inhibiting the enzymes responsible for cross-linking adjacent molecules in the peptidoglycan layer. The β-lactam antibiotics bind to these enzymes, collectively known as penicillin-binding proteins (PBPs) and prevent them from forming cross-links.
Figure 10 shows what happens when a β-lactam antibiotic, in this case penicillin, binds to an active PBP.
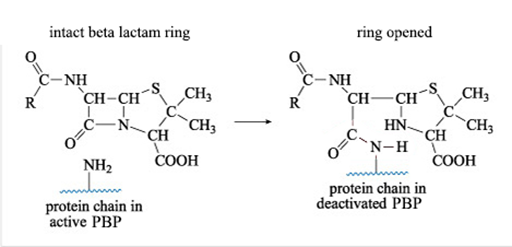
Figure 10 Reaction of penicillin with a PBP. The –NH2 side chain of the PBP reacts with the β-lactam ring of penicillin to form a new side chain. This reaction releases the strain in the β-lactam ring, which remains open.
Show description|Hide descriptionThis is the equation for the reaction of penicillin with a side chain amine group, –NH2, on a protein molecule. The bond between the nitrogen and the carbonyl carbon atom of the β-lactam ring is broken, and this carbon forms an amide, –CONH–, linkage with the amine group on the protein. At the same time, one of the protein’s amine hydrogen atoms is transferred to the N atom that was in the β-lactam ring. The reaction results in deactivation of the protein.
The reaction shown in Figure 10 results in a new PBP side chain which is much larger than the original –NH2 group and effectively deactivates the PBP.
By interfering with the formation of peptidoglycan cross-links β-lactam antibiotics weaken the cell wall. In addition, repairs cannot be made to the cell walls, allowing the osmotic pressure inside the cells to increase, eventually leading to lysis.
Later in the module you will learn more about how disrupting the interaction between β-lactam antibiotics and PBP contributes to antibiotic resistance mechanisms.
The glycopeptides (e.g. vancomycin) are another group of antibiotics that inhibit cell wall synthesis, but by a different mechanism.