5.3 International efforts
In Section 3.3 you learned that the UN General Assembly gathered in September 2016 to discuss the problem of AMR.
The 193 member states committed to work collaboratively to take worldwide action to control AMR by signing the UN Declaration on AMR. All the declaration signatories have agreed that drug-resistant infections must be tackled as a priority and have committed to:
- developing surveillance and regulatory systems on the use and sales of antimicrobial medicines for humans and animals
- encouraging innovative ways to develop new antibiotics and improve rapid diagnostics
- raising awareness among health professionals and the public on how to prevent drug-resistant infections.
By taking this course and discussing what you have learnt with colleagues, friends and family, you are already helping to raise awareness of AMR.
AMR surveillance is critical to tackling AMR, and is the backbone of these efforts. In AMR surveillance and you you learned that AMR surveillance is the ongoing collection, analysis, interpretation and dissemination of data related to AMR.
- At a global level, AMR surveillance provides quantitative data about the spread of resistant strains of bacteria, revealing trends and potentially identifying hotspots of resistant infections.
- At a regional level, surveillance data informs intervention priorities and helps to identify gaps in service delivery.
- At a national level, data guides planning and resource-allocation, and informs policies and responses to patterns and trends.
AMR surveillance data are important to inform policy-makers and decision makers at all levels.
Let’s look at two surveillance programs in the framework of the Global Action Plan on AMR supported by the tripartite collaboration:
Use of antimicrobials in animals
As you learned in Section 4.3, there is limited information available on use of antimicrobials in animals. The World Organisation for Animal Health (
In the fourth round of data collection, 152 member states submitted complete reports, of which 118 included quantitative data on animal use. However, based on challenges faced by countries to collect quantitative data on antimicrobial use in animals, the OIE advises caution in interpretation and use of quantitative data. Based on data reported to the OIE from 92 countries, the global estimate of antimicrobial agents used in animals in 2016 adjusted by animal biomass was 144.39 mg/kg (Góchez, 2019; OIE, 2020).
GLASS, coordinated by the WHO
In 2015, the
The Global Antimicrobial Resistance Surveillance System (
Activity 25: The Global Antimicrobial Resistance Surveillance System (GLASS)
Take a look at Figure 10, taken from the 2020 GLASS report (WHO, 2020):
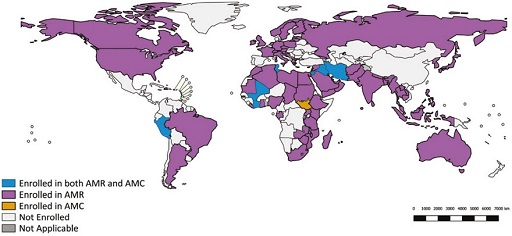
- Is your country enrolled in GLASS?
- If so, is it enrolled for AMR or antimicrobial consumption surveillance, or both?
You will learn more about GLASS in the module An overview of national AMR surveillance.
5.2 One Health