5.3 Setting up national surveillance systems – the WHO’s GLASS
Obtaining reliable, accurate national data requires standardised approaches to sample collection, transport, storage, analysis and reporting at local sites. Similarly, national data must be reliable and comparable if they are to be integrated into regional or global surveillance reports, and examples such as EARS-NET and ReLAVRA are testament to this commitment.
To support international AMR surveillance, the WHO has developed the
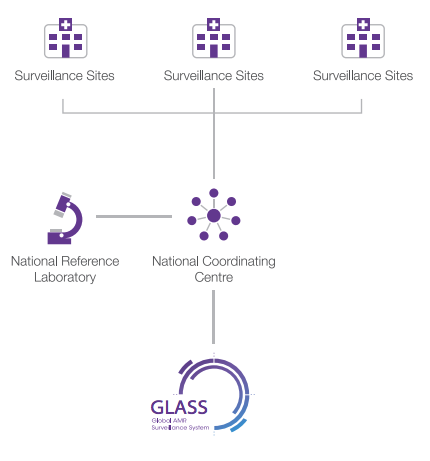
According to GLASS, a national surveillance system should comprise three core components (WHO, 2020) (Figure 8):
- a national coordinating centre
- a national reference laboratory
- selected AMR surveillance sites.
In addition to the core components, GLASS prompts national coordinating sites to standardise their reporting of antibiotic susceptibility testing (AST) data in line with internationally recognised standards – either the
From your study of earlier sections suggest some of the likely pathogens of interest.
- Escherichia coli
- Staphylococcus aureus
- Acinetobacter baumannii
- Klebsiella spp.
- Pseudomonas aeruginosa
- Enterobacteriales
- Enterococcus spp.
- Klebsiella pneumoniae
- Acinetobacter species
- Streptococcus pneumoniae
- Staphylococcus aureus
- Salmonella spp.
- Shigella spp.
- Vibrio cholerae
- Streptococcus pneumoniae
- Neisseria meningitidis
- Haemophilus influenzae
5.2 Multinational surveillance systems