6 Diagnostic stewardship and GLASS
Data collected in the course of clinical diagnostic testing are invaluable in informing institutional treatment guidelines based on local resistance epidemiology. In addition to the test results themselves, demographic information such as age and gender, and how long the patient was in hospital before sampling, is a major source of data that can be used to model the patterns and transmission of pathogens, whatever their AMR pattern.
For the hospital and local healthcare facilities, local or national resistance surveillance data will inform the choice of empirical treatment. However, bacterial resistance patterns are not restricted to geographical regions, but can instead arise and travel rapidly around the world. For this reason, the WHO has implemented the
Figure 5 shows the relationship between laboratory results generated for individual patient care and surveillance data that are used to inform empirical treatment recommendations and AMR control strategies. It illustrates how these can be coordinated at a national level and then fed into GLASS.
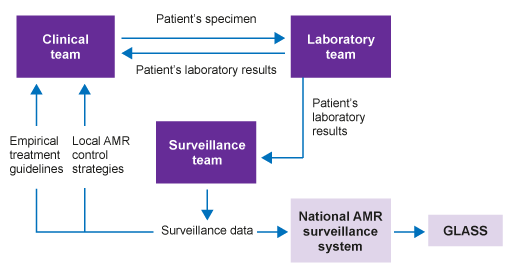
Participation in GLASS requires the use of standard methods for pathogen identification, and AST using CLSI and EUCAST guidelines. It also requires data to be collected at a national level, with both a national co-ordination centre and reference laboratory.
Diagnostic stewardship is a key component for surveillance centres, which supply data to the national system. Guidance for diagnostic stewardship in GLASS is provided by the WHO (2016) and in the associated webinars (Folkhälsomyndigheten Sverige, 2019a, 2019b).
GLASS initially targets only four specimen types: for each one, GLASS provides a list of priority pathogens to be reported. These are reviewed on a regular basis. In addition, GLASS specifies combinations of pathogen and antimicrobials prioritised for surveillance.
It is important to note that GLASS does not require countries to contribute data on every sample, or monitor every priority pathogen. The choice of samples and pathogens can be made in accordance with national priorities and existing capabilities; blood samples are a good starting point for introducing diagnostic stewardship to the standard required.
5.3 Summary reports on a periodic basis



