5.3 International efforts
In Section 3.3 you learned that the UN General Assembly gathered in September 2016 to discuss the problem of AMR.
The 193 member states committed to work collaboratively to take worldwide action to control AMR by signing the UN Declaration on AMR. All the declaration signatories have agreed that drug-resistant infections must be tackled as a priority and have committed to:
- developing surveillance and regulatory systems on the use and sales of antimicrobial medicines for humans and animals
- encouraging innovative ways to develop new antibiotics and improve rapid diagnostics
- raising awareness among health professionals and the public on how to prevent drug-resistant infections.
By taking this course and discussing what you have learnt with colleagues, friends and family, you are already helping to raise awareness of AMR.
The UN held a second high-level meeting on antibiotic resistance eight years after the first, during the General Assembly in September 2024. Follow this link [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] to read more about the decisions taken at this meeting, including a target of reducing human deaths associated with AMR by 10% by 2030.
AMR surveillance is critical to tackling AMR, and is the backbone of these efforts. In AMR surveillance and you you learned (or will learn) that AMR surveillance is the ongoing collection, analysis, interpretation and dissemination of data related to AMR.
- At a global level, AMR surveillance provides quantitative data about the spread of resistant strains of bacteria, revealing trends and potentially identifying hotspots of resistant infections.
- At a regional level, surveillance data informs intervention priorities and helps to identify gaps in service delivery.
- At a national level, data guides planning and resource-allocation, and informs policies and responses to patterns and trends.
AMR surveillance data are important to inform policy-makers and decision makers at all levels.
Let’s look at two surveillance programs in the framework of the Global Action Plan on AMR supported by the quadripartite collaboration:
Use of antimicrobials in animals
As you learned in Section 4.3, there is limited information available on use of antimicrobials in animals. The
In the eighth round of data collection, 152 member states submitted complete reports, of which 129 included quantitative data on animal use. However, based on challenges faced by countries to collect quantitative data on antimicrobial use in animals, the WOAH advises caution in interpretation and use of quantitative data. Based on data reported to the WOAH from 81 reliably reporting countries, the global estimate of antimicrobial agents used in animals in 2021 adjusted by animal biomass was 109.7 mg/kg (WOAH, 2024).
The WOAH provides more information about antibiotic use in animals and its control in ANIMUSE, a global database on ANImal antimicrobial USE.
GLASS, coordinated by the WHO
In 2015, the
The Global Antimicrobial Resistance Surveillance System (
- GLASS EAR and GLASS FUNGI: Focused surveillance activities for emerging resistance (GLASS EAR) and fungi (GLASS FUNGI).
- EGASP: Sentinel gonorrhoea surveillance of men with urethral discharge and suspected urogenital infections.
- Tricycle: An integrated multi-sector surveillance programme based on the extended-spectrum beta-lactamase (ESBL)-Escherichia coli.
- PPS-AMU: A method for the conduct of point prevalence surveys (PPS) of antibiotic use (AMU) at the hospital level.
- Studies estimating the public health impact of AMR.
You can learn more about some of these methods and programmes in other courses in this programme.
As of October 2024, 140 countries, territories and areas are enrolled in GLASS (WHO, 2024). Not all these countries will have completely functional surveillance systems, although they should all be developing them: countries in GLASS may be enrolled in systems for AMR surveillance, antibiotic consumption surveillance, or both.
Activity 25: The Global Antimicrobial Resistance Surveillance System (GLASS)
Take a look at Figure 10, taken from the WHO GLASS website:
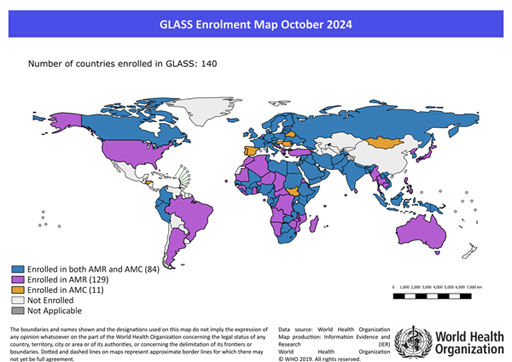
- Is your country enrolled in GLASS?
- If so, is it enrolled for AMR or antimicrobial consumption surveillance, or both?
You will learn more about GLASS in the course An overview of national AMR surveillance.
5.2 One Health