2 The diagnostic workflow in the clinical microbiology laboratory
Clinicians make diagnoses based on the patient’s history and risk factors, physical findings on examination and the results of diagnostic testing (such as imaging, laboratory tests, etc.).
Laboratory diagnostic testing can be broken down into three stages:
- Pre-analytical: Test-related decision-making and specimen collection.
- Analytical: The test itself and any associated laboratory practices, including test methods, microscopy, culture and identification, antimicrobial susceptibility testing (AST), and protocol validation.
- Post-analytical: Reporting and use of laboratory data, such as selective reporting of antimicrobial susceptibility data to encourage the use of narrower spectrum agents.
From the clinical perspective, diagnostic stewardship focuses on stages 1 (pre-analytical) and 3 (post-analytical); most clinicians are not involved in the actual test performed in laboratory (stage 2). The overall value of the test itself relies on good quality at all stages, not just at the analytical stage.
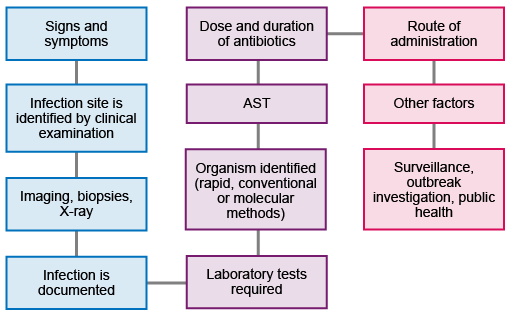
Figure 2 shows the workflow from initial presentation of the patient through to the collection and use of the data to inform further action and strategy. Not all patients will require all the steps: for example, not everyone will require imaging or biopsies, and not all infections will need public health interventions.
Key processes of laboratory diagnostic testing in the pre-analytical, analytical and post-analytical steps are shown in Table 1.
| Stage | Process |
|---|---|
| Pre-analytical |
|
| Analytical |
|
| Post-analytical |
|
The workflow through the laboratory, from test selection through analysis to reporting, is greatly assisted by the use of a Laboratory Information and Management System (LIMS). This is a piece of software that automates and manages laboratory procedures, samples and associated data, and is used in most modern labs. In 2020 a global think tank, the Surveillance and Epidemiology of Drug Resistant Infections Committee (SEDRIC), identified a lack of suitable LIMSs as an important reason for poor quality AMR data from LMICs. SEDRI-LIMS (Global Lab etools, n.d.), a microbiology focused open-source system, was developed as a response to this need and is now beginning to be rolled out across sites in Africa, South America and South-East Asia.
This versatile system can be used in a wide range of organisations, and the software can be run from a single laptop, a network or the Cloud. Examples of situations where it has been deployed (Global Lab etools, n.d.) include:
- a single laptop in a small laboratory in Sierra Leone where Internet and power supplies are unreliable and many systems are paper-based
- a children’s hospital in Cambodia
- a Spanish-language version in a chain of hospitals in Argentina.
Sections 3–5 will consider the three stages of the pathway in more detail. The personnel involved in each stage will depend on the institution, but will usually include physicians, laboratory staff, infection prevention and control teams, and pharmacists. Where available, clinical microbiologists (medical doctors who have specialised in microbiology) should oversee and coordinate the pathway.
1.3 Finding the ‘sweet spot’