Resource 4: Reacting acids and metals
![]() Background information / subject knowledge for teacher
Background information / subject knowledge for teacher
Reacting metals and acids
You will of course know that metals differ in their reactivity. This is illustrated by the reactivity series, which is usually displayed as a league table with the most reactive metal at the top. Usually we regard a metal as reactive if, when it is added to acid, it produces lots of bubbles and the temperature of the acid solution increases.
Common metals for reacting with acids (in order of reactivity) would be magnesium, zinc, iron, tin and copper; i.e. one from the top, three from the middle and one from the bottom of the reactivity series. Magnesium is reactive enough to show significant effervescence without being dangerous, copper is unreactive but not expensive as are silver and gold.
All of the metals above hydrogen in the reactivity series will, produce hydrogen gas from acids. However, the reactions become progressively less vigorous as you go down the reactivity series. The choice of acid is usually hydrochloric acid of concentration 1 mol dm-3 but some metals react better with sulfuric acid; e.g. zinc. Iron reacts but only very slowly. Tin also reacts so slowly that it is difficult to see the reaction. Concentrated acids should not be used.
| magnesium | + | hydrochloric acid | → | magnesium chloride | + | hydrogen |
| Mg(s) | + | 2HCl(aq) | → | MgCl2(aq) | + | H2(g) |
| zinc | + | sulfuric acid | → | zinc sulfate | + | hydrogen |
| Zn(s) | + | H2SO4(aq) | → | ZnSO4(aq) | + | H2(g) |
| iron | + | hydrochloric acid | → | iron(II) chloride | + | hydrogen |
| Fe(s) | + | 2HCl(aq) | → | FeCl2(aq) | + | H2(g) |
| tin | + | hydrochloric acid | → | tin(II) chloride | + | hydrogen |
| Sn(s) | + | 2HCl(aq) | → | SnCl2(aq) | + | H2(g) |
Copper is below hydrogen in the reactivity series and will therefore not displace dilute acid so there is no reaction.
As you can see from the above equations the reactions produce salts. You can introduce your pupils to the reactions of metals with acids in one lesson to establish the fact that some displace hydrogen from acids, by collecting and testing for the gas with a lighted splint, and then, perhaps, extending their understanding by isolating one of the salts in the next lesson. For example, zinc granules will react with dilute sulfuric acid (0.5 mol dm-3) to produce zinc sulfate which can then be isolated by crystallisation by evaporating off the excess solvent in an evaporating basin as shown in the diagram below. If you decide to use zinc and sulfuric acid to make a salt, then adding a few drops of copper sulfate will speed the reaction up.
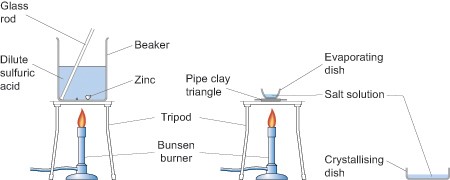
Health and safety
Obviously this experiment involves heating so great care needs to be taken when handling apparatus which should be left for a sufficient time after heating for them to have cooled down.
Arrange the class so that pupils work in pairs or small groups. No pupils should be seated if they or members of their group are heating anything.
When heating the evaporating basin the Bunsen flame should be blue but the gas tap adjusted so that there is a low flame for gentle heating. When the water level in the basin has been reduced by about a half place a glass stirring rod in the solution and then hold it up to cool. If small colourless crystals begin to form on the rod stop heating and allow the basin to cool naturally. If no crystals form continue heating until they do. Do not continue heating past the point at which crystals are observed at the edge of the solution.
1. 0 mol dm-3 HCl is a low hazard at this concentration.
0.5 mol dm-3 H2SO4 is an irritant at this concentration.
Resource 3: Neutralisation Circus



