10 Mass spectroscopy
Mass spectrometry can be used to provide a ‘fingerprint’ of the different pesticides or compounds. In mass spectrometry the molecules are bombarded with high energy electrons to cause some of the molecules to break up into smaller positively charged particles, or fragment ions. All the ions are accelerated and then subjected to a magnetic field that bends (deflects) the ions by different amounts depending on their mass or, strictly speaking, their mass to charge ratio (m/z). Light ions are deflected most, while heavy ones tend to go straight on. A detector measures how much ions are deflected which is used to produce a mass spectrum.
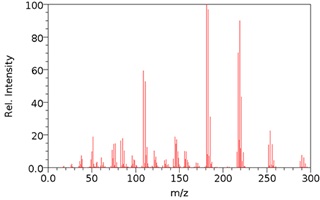
The mass spectrum of the pesticide lindane is shown in Figure 7.
What is the relative molecular mass of lindane, C6H6Cl6?
Answer
Considering the mass of its constituent atoms the relative molecular mass of lindane can be calculated as:
(6 × 12) + (6 × 1) + (6 × 35.5) = 291 atomic mass units
Although a small peak due to the molecular or parent ion is present, the mass spectrum itself is complex, reflecting the different possible fragment ions that are produced. Rather than analysing the peaks in the mass spectrum of each pesticide and building up the structure in that way, the spectrum can be compared with a ‘library’ of mass spectra of a range of different pesticides/related molecules. This is what you will be doing in this investigation to identify any pesticides present in your samples.
9 Gas chromatography in practice continued