4 The chemical structure of pesticides
Examples of the pesticides that you will be analysing are given in Table 1.
| Pesticide | Molecular formula | Structure | Information Footnotes a |
| Aldrin | C12H8Cl6 | 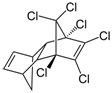 | pesticide (banned but restricted use as a local insecticide) |
| Dieldrin | C12H8Cl6O | 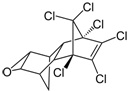 | insecticide (banned but restricted use for agricultural purposes) |
| Dichlorodiphenyltrichloroethane (DDT) | 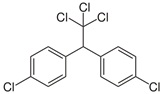 | pesticide (banned but restricted use for malaria control) | |
| Endosulfan | C9H6Cl6O3S | 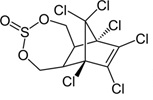 | insecticide (banned only recently, still in use in China and India) |
| Endrin | C12H8Cl6O | 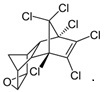 | insecticide and rodenticide (banned) |
| Hexachlorocyclohexane (αHCH) | 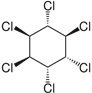 | pesticide (banned) | |
| Lindane (γ-HCH) | C6H6Cl6 | 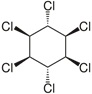 | insecticide (banned but restricted pharmaceutical use for treatment of lice and scabies) |
| Heptachlor | 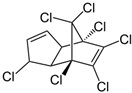 | insecticide (banned but restricted use use as a termiticide) | |
| Methoxychlor | 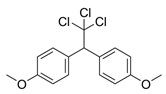 | insecticide (banned in US and EU) | |
| Pentachloronitrobenzene (PCNB) | C6Cl5NO2 | 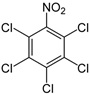 | fungicide |
Footnotes
Footnotes a Banned status according to the United Nations Treaty (known as the Stockholm Convention on Persistent Organic Pollutants), which has been signed by the majority of countries. One notable exception is the USA, which has its own list of banned substances covering many of the same pesticides.Look at the pesticide structures in Table 1. Most of these probably look more complicated than the sorts of things you’ve seen before but they follow just the same principles as simpler molecules. To simplify them carbon and hydrogen atoms aren’t labelled in these skeletal structures, but we need to remember them. To get a feel for this, Figure 3 shows two alternative ways of depicting a benzene ring, with the structure on the right corresponding to the method used for the pesticide structures in the Table.
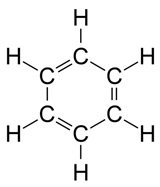 | 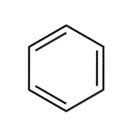 |
3 What you’ll be doing in this investigation
