Appendix 2: Teacher notes - outputs of the lesson (Exploring the properties of metal ions using a hot flame)
Appendix 2: Teacher notes - outputs of the lesson (Exploring the properties of metal ions using a hot flame)
Syllabus sections addressed by this lesson
Teaching Syllabus for Chemistry (SHS 1–3)
SHS1 Section 2 Unit 3 Periodicity
- 2.3.1 Relate the position of an element in the periodic table to its atomic number and electron configuration.
- 2.3.4 Distinguish between the terms ‘group’ and ‘period’ (example Alkali metals)
The following Practical and Experimental Skills in Chemistry will be developed:
- Observations
- Drawing
- Interpretation
- Reporting
- Conduct in Laboratory
Teaching Syllabus for Physics (SHS 1–3)
SHS1 Section 6 Unit 1 Models of the Atom and Atomic Structure
- 6.1.2 Explain the existence of quantized energy levels in an atom
- 6.1.3 Describe the types of spectra and their uses
The following Practical and Experimental Skills in Physics will be developed:
- Make observations
- Design and conduct investigations
- Analyse and interpret results of scientific investigations
- Communicate and apply the results of scientific investigations
Setting up equipment
To carry out this lesson, students can work on a computer individually or in pairs. Students who are not able to distinguish between different colours should work with another person.
Students need their laboratory notebooks, pen, pencil (for diagrams) and ruler.
Organising the lesson
The estimated lesson time is 60 minutes, but it could be completed in less time by reducing the amount of writing required in laboratory notebooks, or omitting Activity 2 to identify the mystery samples. It could be split between two shorter lessons by covering the background theory and Method in Lesson 1 and carrying out the experiment and recording the results, discussion and conclusions in Lesson 2. Computers would be needed for both lessons.
The lesson could be extended in the following ways:
- Produce a presentation about the activity for students who have not studied this topic (using presentation software or on paper).
- Produce a one-page summary of the difference between continuous and line spectra, with examples and illustrations.
Laboratory notebook record for practical on using flame tests to identify metal ions
Title: Using flame tests to identify metal ions
Aim: To identify elements using flame colours and emission line spectra.
Introduction
When metal ions are heated in a flame they produce a characteristic flame colour and emission line spectrum. A spectroscope can be used to observe the emission line spectrum, from which we can identify the elements present. The light is produced when electrons move from an excited state to a lower energy level.
Method
1. Step by step method
- Turn on gas tap and light Bunsen burner.
- Open air hole of Bunsen burner to obtain a blue flame.
- Pick up nichrome wire and clean it.
- Dip the nichrome wire into one of the metal compound samples.
- Place the sample in the centre of the blue flame and observe the colour of the flame.
- Use the spectroscope to observe the line emission spectrum.
- Record your results in a table.
- Wash the nichrome wire in the wash beaker and repeat for the next sample.
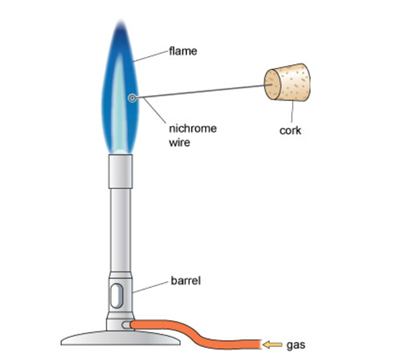
Figure: Components of a Bunsen burner and position of the nichrome wire for flame tests.
Results
Known metal ion salts
|
Element |
Chemical symbol |
Colour of flame |
Description of the spectrum |
|
Sodium |
Na |
Yellow |
Two bright yellow lines, one weaker blue line, one weaker green line and one weaker red line
|
|
Lithium
|
Li |
Magenta red |
Bright orange/red line and weaker red and blue lines.
|
|
Potassium
|
K |
Purple/ |
Yellow, green, blue and purple lines.
|
|
Copper
|
Cu |
Green/ |
Bright blue/green lines, with a pair of yellow lines.
|
|
Strontium
|
Sr |
Crimson red |
Two bright red lines, with many green and blue lines.
|
Mystery metal ion salt samples
|
Sample |
Colour of flame |
Description of the spectrum |
Elements present |
|
1
|
Green/blue |
Bright blue/green lines, with a pair of yellow lines.
|
Copper
|
|
2
|
Purple/pink/lilac |
Yellow, green, blue and purple lines
|
Potassium |
Note: mystery sample 1 does not show the blue colour of the copper metal ion salt in solution – this was done so that the student has to interpret the flame colour and spectrum in order to identify the metal ion. In fireworks, gunpowder is mixed with compounds of different metals to produce different colours. Red fireworks contain strontium and lithium compounds. Blue fireworks contain copper compounds. Yellow fireworks contain sodium compounds. Purple fireworks contain copper and strontium compounds.
Previous: Appendix 1 Next: Lesson: Exploring the properties of metal ions using a hot flame