Background (Exploring the properties of metal ions using a hot flame)
Background
Have you ever wondered why fireworks (Figure 1) have different colours? Fireworks contain gun powder (a source of heat) and metal ions, and by the time you have finished this practical lesson you will be able to explain the science behind the colour of
fireworks.

Figure 1. Fireworks of different colours
In this lesson you will observe the colours produced by different metal ions in a hot flame and describe the emission line spectrum (plural, spectra) produced. You will explain your observations in terms of atomic structure.
Atoms and energy levels
The behaviour of electrons in an atom determines many of its characteristic properties. The electrons in atoms have specific energies. This is quantisation of energy. Each element has its own characteristic energy levels.
The simplest atom, in terms of its atomic structure, is the hydrogen atom. It consists of a single proton at its centre and one electron as shown in Figure 2.

Figure 2. The atomic structure of a hydrogen atom consisting of one proton and one electron.
The hydrogen atom can exist in one of 7 energy states and this can be represented by an energy level diagram, (Figure 3(a)). The lowest energy level is E1 and the highest level is E7. Another way to think about this is to imagine the rungs of a ladder with the proton fixed at the bottom but the electron free to climb the ladder; the higher it gets, the higher its energy level (Figure 3(b)).
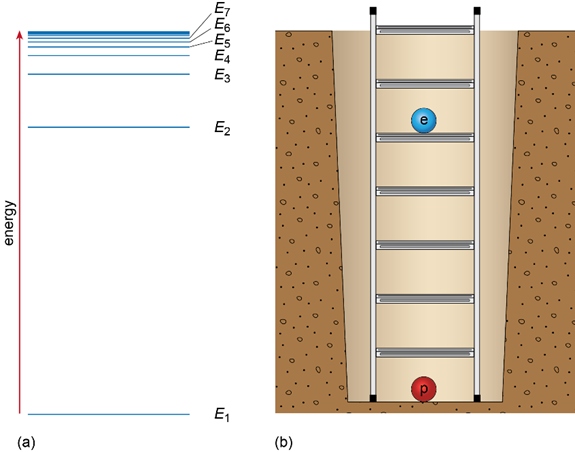
Figure 3. (a) The energy level diagram of hydrogen. Energy increases moving up this diagram. (b) The energy level diagram is like the rungs of a ladder sunk into a deep pit. The higher the energy level, the greater the separation between the proton and the electron.
When an atom is heated, its electrons move from their ground state (E1) to a higher energy level and are said to be in an excited state (e.g., E2 or higher). When the electrons return to a lower energy state or the ground state, energy is emitted in the form of light. The ‘packets’ of light produced by individual atoms are called photons. The change of an electron from one energy level to another is called a transition.
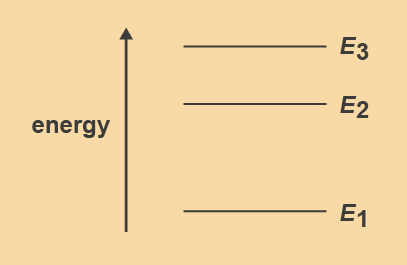
Figure 4. Energy level diagram with three levels
|
A hypothetical atom, shown in Figure 4, has three energy levels (E1, E2 and E3). How many different transitions are possible that would result in the emission of photons? |
The relative separation between two energy levels determines the energy of the photons emitted. In Figure 4 the energies of emitted photons are: E3 to E1, E3 to E2 and E2 to E1, respectively. Each transition produces light of a particular wavelength.
Every element produces light of several specific wavelengths and when a metal ion is heated in a hot flame this gives the flame of each element a characteristic colour. The more energy the photons have, the shorter their wavelength.
|
Yellow light has photons of lower energy than green light. Which colour has the longer wavelength? |
When sodium ions are placed in a hot flame, the flame burns with a distinctive yellow colour, as shown in Figure 5.

Figure 5. Flame test for sodium ions.
To answer the following questions, you may need to refer to a Periodic Table.
|
What is the chemical symbol for sodium? |
|
What Group of the Periodic Table is sodium in, and what other elements are in the same group? |
In this practical activity you will examine behaviour of some Alkali metal ions and other metals.
Spectral lines: an atom’s signature
When a beam of white light from the Sun is passed through a prism it is broken up or dispersed, into a range of colours that form a pattern similar to a rainbow. Such a band of colours is called a spectrum (plural, spectra). The spectrum of white light or sunlight forms an uninterrupted band of colours known as a continuous spectrum, as shown in Figure 6.
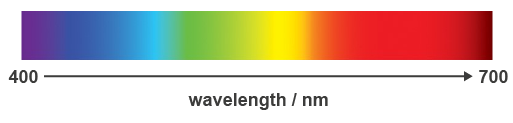
Figure 6. A continuous spectrum from a beam of white light or sunlight.
|
The spectrum in Figure 6 goes from violet to red. Which of the following two colours has the longer wavelength – blue or yellow? |
A similar technique can be used to study the light emitted by atoms and ions when they are heated in a flame. The spectrum produced is different from the continuous spectrum for sunlight as it consists of very narrow lines of specific colours. Figure 7 shows the emission line spectrum for sodium ions.

Figure 7. Emission line spectrum for sodium ions in the visible region.
|
Some lamps, such as some road lamps, contain sodium vapour in an excited state and produce yellow light. Why does the light appear yellow when there are lines of other colours in the spectrum? |
Spectroscopy: studying spectral signatures of metals
The technique for identifying which metal atoms are present in a sample of material by analysing the emission line spectrum is called spectroscopy. Scientists often want to discover which elements may be present in a sample of material. They may also want to make compounds that produce light of specific colours, for instance in fireworks.
In this practical activity you will observe the flame colours for lithium, potassium and other metallic elements and examine them using a spectroscope(see Figure 8). This is an instrument that contains prisms and produces a spectrum of light in the form of a set of coloured lines.
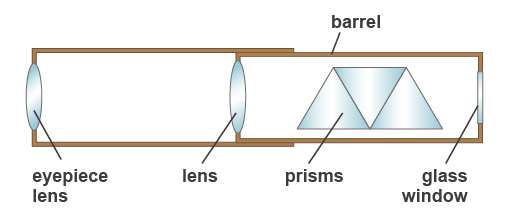
Figure 8: Main components of a hand spectroscope
|
What type of spectrum will you observe using the spectroscope to examine the colour of the flames? |
Previous: Lesson objectives Next: Practical activity
