Background (Qualitative chemical analysis: Chemical tests for cations)
Background
Ionic compounds
The elements in the Periodic Table can be divided in two groups (Figure 1):
- metals (including sodium, calcium, copper, aluminium and iron)
- non-metals (including oxygen, carbon, hydrogen, nitrogen, sulfur and chlorine).

Figure 1. Periodic Table showing metals and non-metals.
When a metal atom comes into contact with a non-metal atom, the metal atom has a tendency to lose an electron (some metals atoms lose more than one) and donate it to the non-metal atom. The atoms losing or gaining electrons become charged particles called ions.
|
What is an electron and what charge does it carry? |
Metal atoms form positively charged ions, also known as cations. And the non-metal atom that receives the extra electron will become negatively charged because it has gained a negatively charged electron. These negatively charged ions are also known as anions. Cations and anions are attracted to each other by their opposite charges; this type of interaction is known as an ionic bond.
Figure 2 shows the ions formed when sodium interacts with chlorine to form the ionic compound sodium chloride (NaCl), known as common table salt. Electrons are shown orbiting the atomic nucleus in electron shells. The sodium atom loses one electron to form a positively charged sodium ion with chemical symbol Na+. The electron that the sodium atom loses is transferred to the chlorine atom, which becomes a negatively charged chloride ion (with chemical symbol Cl-). Note that the charged of an ion is shown as a superscript plus ‘+’ or superscript minus ‘-’ sign to the right of the chemical symbol.
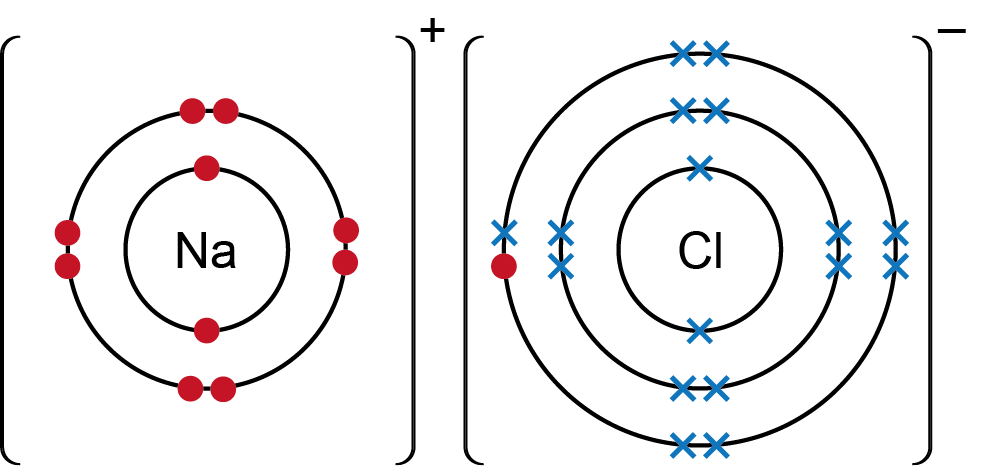
Figure 2. Sodium chloride (NaCl) is an ionic compound formed by ionic bonding between a positively charged sodium ion (written as Na+) and a negatively charged chloride ion (written as Cl-). Electrons of the sodium atom are represented by red dots and electrons of the chlorine atom are represented by blue crosses.
Chemists use the term ‘salt’ to refer not only to sodium chloride, but also to any ionic compound formed in that way. The general rule is that ionic compounds form when metals bond with non-metals.
|
In calcium chloride (CaCl2), the calcium forms a positive ion by losing two electrons (one to each of the two chlorine atoms). What is the charge on the calcium ion and on each chloride ion? |
Not all ions are formed from a single charged atom. Molecular ions are larger charge particles consisting of a group of atoms that has lost or gained some electrons. For example, sodium hydroxide (NaOH) is an ionic compound formed by ionic bonding between a positively charged sodium ion (Na+) and a negatively charged hydroxide ion (OH-). A hydroxide ion is a negatively charged group in which oxygen shares an electron with hydrogen (to form a covalent bond between the oxygen and hydrogen atoms), but also gains an electron from the sodium atom (Figure 3).

Figure 3. Sodium hydroxide (NaOH) is an ionic compound formed by ionic bonding between a sodium ion (Na+) and a hydroxide ion (OH-). Electrons of the sodium atom are represented by red dots, electrons of the chlorine atom are represented by blue crosses and the electron of the hydrogen atom is represented by a black dot.
|
What is the main difference between ionic and covalent bonds? |
Other examples of ‘molecular ions’ you will come across are:
- The nitrate ion (NO3-) which has an overall charge of -1 and forms ionic compounds such as sodium nitrate (NaNO3) where the nitrate group gains an electron from a sodium atom.
- The sulfate ion (SO42-), which has an overall charge of -2 and forms ionic compounds such as sodium sulfate (Na2SO4), which has an overall charge of -2 and forms ionic compounds such as sodium sulfate (Na2SO4) where the sulfate group gains one electron from each of the sodium atoms.
|
The carbonate ion forms ionic compounds such as sodium carbonate (Na2CO3). Which is the overall charge of the carbonate ion? |
Water as a solvent
In chemistry, a solution is a homogeneous (uniform) mixture in which a substance (known as the solute) dissolves in another substance (known as the solvent). A solvent is usually a liquid, but it could also be a gas. Water (H2O) is an excellent solvent and it is also called the universal solvent.
Although a water molecule has a neutral charge overall, it has some areas that are slightly more negative (the oxygen atom) and some areas that are slightly more positive (the hydrogen atoms). The electrons shared between the oxygen and hydrogen atoms in a water molecule are more strongly attracted to the oxygen. The term polarisation is commonly used to describe this partial separation of charge and can be represented as δ- (delta minus) and δ+ (delta plus). In Figure 4, the oxygen atom has a partial negative charge δ- and the hydrogen atoms has a partial positive charge δ+. Due to its bent geometry, water is a polar molecule.
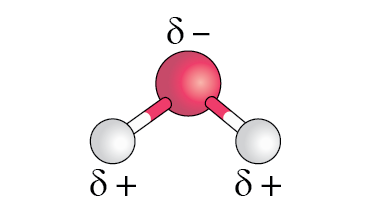
Figure 4. Uneven distribution of charge between the oxygen atom (red) and hydrogen atoms (white) in a water molecule.
The slightly negative area of one water molecule (the oxygen atom) is attracted to the slightly positive area of an adjacent water molecule (the hydrogen atoms) as shown in Figure 5.
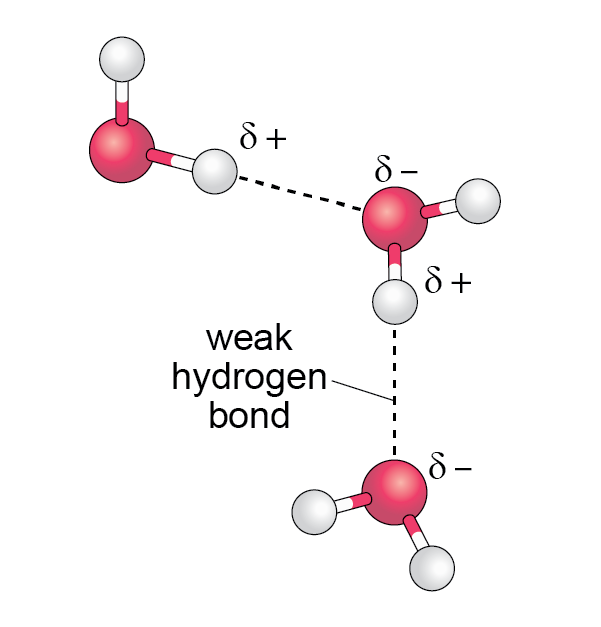
Figure 5. Hydrogen bonds holding adjacent molecules together. The polarised charges are shown.
This attraction between water molecules (known as hydrogen bonds) are a tenth the strength of the covalent bonding within a water molecule between the hydrogen and oxygen atoms. These hydrogen bonds between different water molecules are constantly being broken and reformed as the molecules move around.
Water molecules can also form interactions with many other polar molecules. This is what makes water a good solvent. For example, when salt dissolves in water, the electrical attraction between the Na+ and Cl− ions in the salt crystals is replaced by electrical attraction between the Na+ and Cl− ions and the polarised water molecules. The regular structure of the salt crystals (known as an ionic lattice) then starts to break down as the crystals dissolve in the water.

Figure 6. Solubility of sodium chloride crystal in water.
When salts are dissolved in water, the ions separate, and you should treat them individually. Some ions may react with other ions however few ions may be only spectators to those reactions.
Representing ionic chemical reactions
A chemical equation provides a shorthand way of writing out what happens when the substances present at the beginning of a chemical reaction (the reactants) are transformed into new chemical substances (the products). Similarly, ionic chemical equations can be written for chemical reactions involving ions. Dissolving table salt in water can be represented by:
NaCl(s) → Na+(aq) + Cl-(aq)
In a balanced ionic chemical equation, the number of atoms of each element must be the same on both sides of the equation (the same as when balancing chemical equations for neutral species). Note that the simple ionic equation for dissolving table salt in water, is already balanced with one sodium and one chlorine atom on both sides of the equation. For more complex chemical equations, a good way to see this more clearly is to make a table of the number of atoms present on each side of the equation.
There is an extra check to ensure an ionic chemical equation is balanced; the total charge on each side of the ionic equation must be the same. The total charge on each side of the balanced ionic equation for dissolving table salt in water is zero. Finally, remember to include the state symbols for both reactants and products.
|
Write the balanced chemical equation for dissolving lead nitrate, Pb(NO3)2 in water. |
Precipitation reactions
A precipitation reaction is when substances in solution are mixed, resulting in the formation of a product that cannot be dissolved in the solvent, and so it deposits in solid form from the solution (this deposit is known as a precipitate).
Precipitation reactions are frequently used in qualitative chemical analysis, for example when chemists are tasked with the identification of an ion in an unknown solution.
The chemical equation below represents the precipitation reaction between copper ions and hydroxide ions (both in solution) to form solid copper (II) hydroxide:
Cu2+(aq) + 2 OH-(aq) → Cu(OH)2(s)
The pale blue precipitate Cu(OH)2 formed is used as a test for the identification of copper ion, Cu2+.
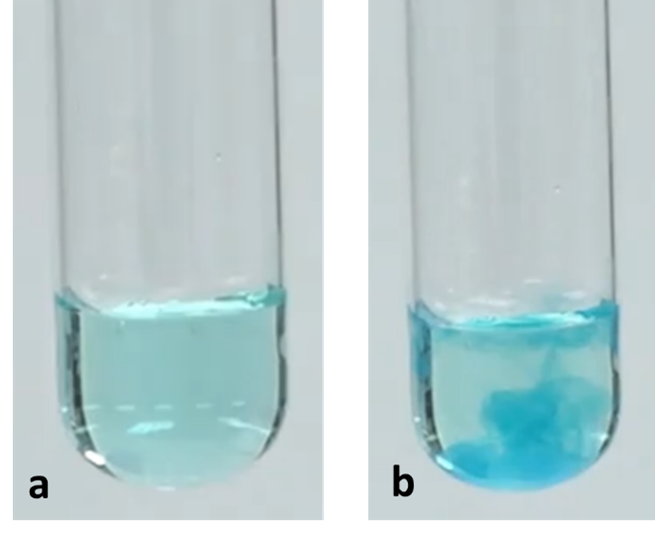
Figure 7. Test tubes containing (a) a diluted solution of copper ions and (b) a solid precipitate of copper (II) hydroxide after adding hydroxide ions to the solution of copper ions.
|
What is the difference between qualitative versus quantitative chemical analysis? |
Reactions of metal ions with sodium hydroxide and ammonia solutions
Sodium hydroxide (NaOH) and ammonia (NH3) solutions are both alkalis (soluble bases), giving hydroxide ions (OH-) in their solutions.
NaOH is a strong base and dissociates completely to form sodium ions and hydroxide ions.
NaOH (aq) → Na+ (aq) + OH- (aq)
On the other hand, NH3 is a weak base, and it does not fully dissociate in water.
NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq)
Note that this is a reversible reaction (represented in the chemical equation with the two arrows but each with just half an arrowhead, the top one pointing right and the bottom one pointing left). In a reversible reaction, the products can react to produce the original reactants again. In this equilibrium the concentration of ammonia is much higher than the concentration of ammonium NH4+ ions and OH- ions.
|
Why do strong bases have a higher pH than weak bases? |
Many metal ions in solution with a charge of n+ (Men+) react with hydroxide ion (OH-) to form different coloured precipitates of their metal hydroxide, Me(OH)n:
Men+(aq) + n OH-(aq) à Me(OH)n (s)
However, when adding more OH- some of these precipitates re-dissolve in excess alkali. For example, you will observe that when a few drops of sodium hydroxide is added to a solution containing aluminium ions, the precipitate formed will re-dissolve when adding excess sodium hydroxide:
Al3+ (aq) + 3 OH-(aq) → Al(OH)3(s)
Al(OH)3(s) + OH-(aq) → Al(OH)4-(aq)
Both observations (precipitates forming with few drops of alkali and any effect of adding excess alkali) are important in qualitative chemical analysis for the identification of metal ions.
The observations with sodium hydroxide solution are usually similar (but not always) to the observations with ammonia solution; any similarities and/or differences can be important clues to the identification of metal ions.

Figure 8. Test tubes containing precipitates of different metal hydroxides.
|
Write the ionic chemical equation for zinc ion (Zn2+) in solution reacting with hydroxide ion (OH-) in solution to form a precipitate of zinc hydroxide. |
Complete versus net ionic chemical equations
The ionic equations discussed in the previous sections only included the ions involved in the formation of a precipitate. This is known as net ionic chemical equation. In this section, you will practice how to write a net ionic equation for precipitation reactions of metal ions with a soluble base.
First, you write the complete balanced molecular chemical equation, representing all the reagents and products in the precipitation reaction. You continue rewriting the chemical equation, substituting any soluble ionic compound with its dissociated ions and obtaining the complete ionic chemical equation. There are some ions on both sides of the complete ionic chemical equation that do not undergo any chemical reaction themselves. These are called spectator ions. Cancelling out the spectator ions leaves you with the net ionic chemical equation.
It takes practice to be able to write balanced ionic chemical equations. Look at the following example for the reaction of copper (II) sulfate solution and sodium hydroxide solution to form solid copper (II) hydroxide:
Copper (II) sulfate + sodium hydroxide → copper (II) hydroxide + sodium sulfate
There are few essential steps to follow:
Step 1: Write the molecular chemical equation using the chemical formula for reactants and products including their state of matter:
CuSO4(aq) + NaOH(aq) → Cu(OH)2 (s) + Na2SO4(aq)
Table – The numbers of atoms in the unbalanced equation
|
Type of atom |
Reactants |
Products |
|
Cu |
1 |
1 |
|
S |
1 |
1 |
|
O |
5 |
6 |
|
Na |
1 |
2 |
|
H |
1 |
2 |
Step 2: Write the balanced molecular chemical equation:
CuSO4(aq) + 2 NaOH(aq) → Cu(OH)2 (s) + Na2SO4(aq)
Table – The numbers of atoms in the balanced equation
|
Type of atom |
Reactants |
Products |
|
Cu |
1 |
1 |
|
S |
1 |
1 |
|
O |
6 |
6 |
|
Na |
2 |
2 |
|
H |
2 |
2 |
STEP 3: Separate the soluble ionic compounds into their ions:
Cu2+(aq) + SO42-(aq) + 2 Na+(aq) + 2 OH-(aq) → Cu(OH)2(s) + 2 Na+(aq) + SO42-(aq)
Note that Cu(OH)2 is a precipitate and it is an insoluble product so it is not separated.
SO42- and Na+ are on both side of the equation so these spectator ions are cancelled out.
STEP 4: Write the net ionic chemical equation for the precipitation of copper (II) hydroxide, showing the actual chemical change (without the spectator ions):
Cu2+(aq) + 2 OH-(aq) → Cu(OH)2(s)
This reaction is used for the identification of copper ion (Cu2+).
|
What is the molecular, ionic and net ionic chemical equation for the precipitation reaction between a solution of aluminum nitrate and a solution of sodium hydroxide to form a precipitate of aluminum hydroxide and sodium nitrate? |
Previous: Lesson objectives Next: Practical activity
