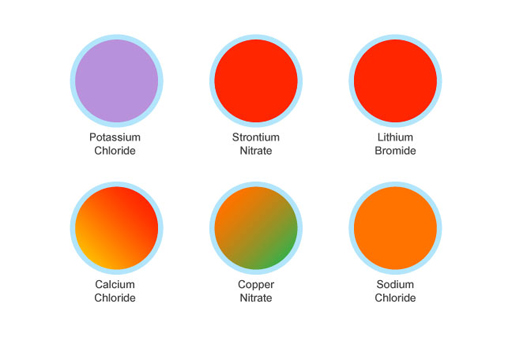
2.1.3 The flame test results
Here are the colours from the flame test. How close were you?
Modern chemists now have a wide range of instruments that identify an unknown chemical or mixture of chemicals. Before these were available, the flame test was important in identifying what these chemicals were. In the flame test, the flame differs depending on what chemicals are present. If an electron in a chemical is heated by a flame, its energy increases. The electron stays in this excited state before ‘relaxing’. When it does relax, it releases a photon. The energy and the colour of the photon depend on what element has been heated.
The colour differences apparent in a flame test can be seen in fireworks, which use different chemical elements, each of which gives a distinct colour. The elements used for fireworks are shown in Table 1.
| Red | Strontium or lithium salts |
| Orange | Calcium salts |
| Gold | Iron |
| Yellow | Sodium salts |
| Green | Barium salts |
| Blue | Copper salts |
| Purple | Mixture of strontium (red) and copper (blue) salts |
| White | Magnesium or aluminium |
2.1.2 The OpenScience Laboratory