Resource 5: Surface tension – information for teachers
![]() Background information / subject knowledge for teacher
Background information / subject knowledge for teacher
If you had never seen a steel needle floating on the ‘thin skin’ of the surface of water, you might have been as surprised as your pupils are likely to be when you demonstrate this. But what is the explanation?
Think it out for yourself. Try to imagine the actual particles of water. In your mind’s eye, see them free to flow and move past and between each other. But they are always being held together by weak forces of attraction. This is happening in all directions. At any one moment in time, any one particle will have neighbours on all four sides (left, right, front and back). There will also be neighbours above and below. Do you get the picture?
Now think of a particle at the surface. It has no particles above it. That leaves the particles at the surface with extra attractive force to spare, so the particles at the surface will hold together more strongly. This creates a tough, fairly strong, temporary skin across the surface. Scientists agree to call this extra pull between surface particles of certain liquids, surface tension.
Water has a much higher surface tension than most other liquids. You might like to try the experiment with other liquids to show this.
Can your pupils think of other examples of surface tension?
Other examples include the shape of water droplets and insects walking on water.

A water droplet – surface tension changes their shape
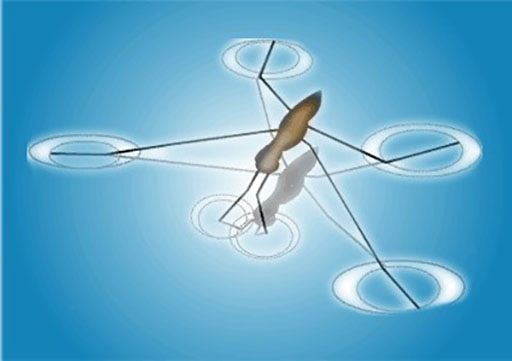
The insect can walk on the water because of the surface tension on the surface of the water.
Original source: http://citt.ufl.edu/ Marcela/ Sepulveda/ html/ en_tension.htm [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] (Accessed 2008)
Resource 4: Making a big book



