Background (Calorimetry: combustion of alcohols)
Background
Chemical reactions and energy changes
In our everyday lives we depend on chemical reactions to provide our energy needs: burning fuels for heating, transport and cooking, and in our bodies where a multitude of chemical reactions keep us alive.
A chemical reaction involves a substance undergoing a change which alters its chemical composition. During a chemical reaction, bonds are broken in the reactants and new bonds are formed in the products. Breaking bonds requires energy whereas making bonds releases energy. The net difference between the bond-breaking and bond-making processes accounts for the energy changes during the chemical reaction.
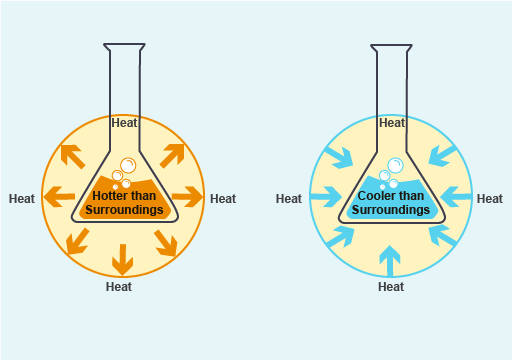
If more energy is released when the bonds are made than is required to break bonds, energy will be released into the environment – these are called exothermic reactions. If the opposite is true and more energy is required to break the reactant bonds than is released when the product bonds are formed, energy must be absorbed from the environment. These are called endothermic reactions. Figure 1 illustrates the difference between exothermic and endothermic reactions in terms of heat energy.
(a) (b)
Figure 1. (a) An exothermic reaction releasing energy (in the form of heat) to surroundings, and (b) an endothermic reaction absorbing energy from surroundings.
|
Think of examples of exothermic and endothermic reactions |
Enthalpy changes of chemical reactions
The chemical energy involved in reaction is called enthalpy change. For a given substance the enthalpy can be thought of as a kind of energy store that provides, or accepts, energy in the form of heat. The symbol for enthalpy of a substance is written as H.
For a chemical reaction the heat released, or absorbed, is a measure of the difference in energy between reactants and products (under the same conditions of temperature and pressure). The enthalpy change of a chemical reaction is written as ΔH, where Δ (the upper-case Greek letter delta) means ‘change of’ and can be calculated as:
ΔH = H(products) − H(reactants)
This equation says that the heat released, or absorbed, by the chemical reaction is equal to the difference between the enthalpy of the products and the enthalpy of the reactants. Since an enthalpy change represents the heat transferred in a chemical reaction, the appropriate SI unit is the joule (J).
In an exothermic reaction, the products have lower energy than the reactants and the reaction releases energy in the form of heat to the surroundings. Therefore, ΔH is negative (ΔH < 0) for an exothermic reaction. Figure 2 shows a schematic representation of the energy changes occurring in both exothermic and endothermic reactions.
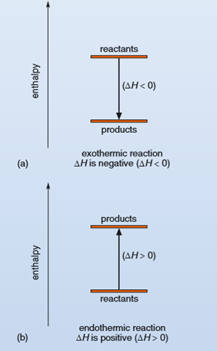
Figure 2. Enthalpy diagrams for (a) an exothermic reaction, where ΔH is negative, and (b) an endothermic reaction, where ΔH is positive. The vertical axes represent enthalpy (H), increasing upwards, and the enthalpies of reactants and the enthalpies of products are represented by different levels on the axes.
Combustion of alcohols
Alcohols are organic compounds containing the hydroxyl (-OH) group that are widely used in our lives and are among the most abundantly produced chemicals in industry.
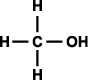
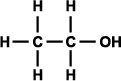
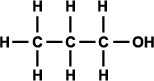
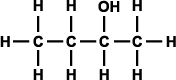
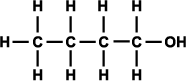
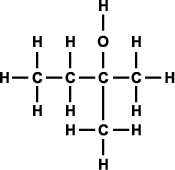
Alcohols can be classified as primary, secondary, and tertiary depending on the position of the hydroxyl group. The hydroxyl group is attached to a carbon with at least two hydrogen atoms in a primary alcohol, to a carbon with only one hydrogen atom in a secondary alcohol, and to a carbon with no hydrogen atoms in a tertiary alcohol (as shown in Figure 3).
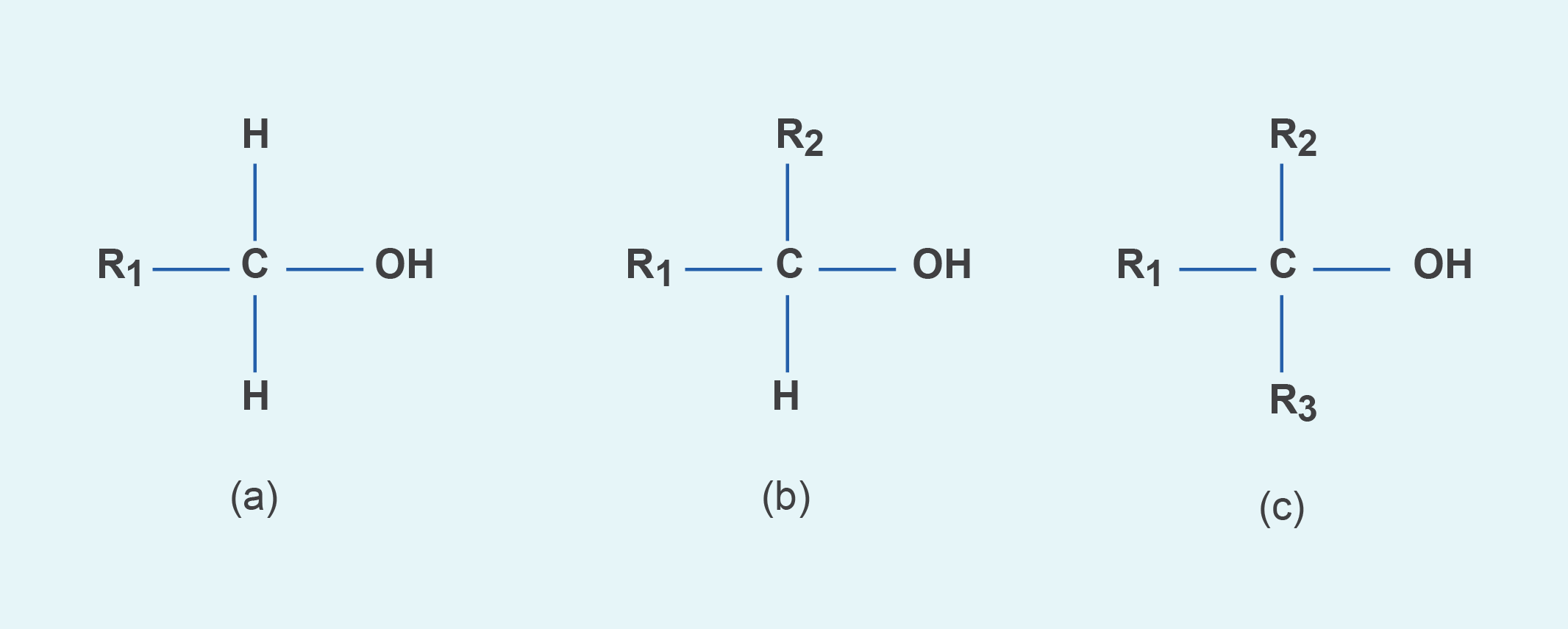
Figure 3. Generic structural formula of (a) primary alcohol, (b) secondary alcohol, and (c) tertiary alcohol. The letter R represents an atom or group of atoms.
You may have come across the term alkanol. Alkanol is a type of alcohol with very specific R groups; the Rs are alkane compounds in alkanols. Alkanes are compounds containing only carbon-carbon single bonds and carbon-hydrogen bonds (examples are methane, ethane, propane). The classification of alcohols shown in Figure 3 also applies to alkanols.
When naming the simple alcohols in this lesson, you should start by identifying the longest straight carbon chain containing the hydroxyl group. The name of the alkane corresponding to the longest chain is used without the final ‘-e’ and adding instead the suffix ‘-ol’. The carbon atoms in the chain should be given a number considering that the carbon attached to the hydroxyl group should have the lowest possible number. If any branch (or group of atoms) is identified of the main chain, you will indicate it in the name of the alcohol as well as its position.
|
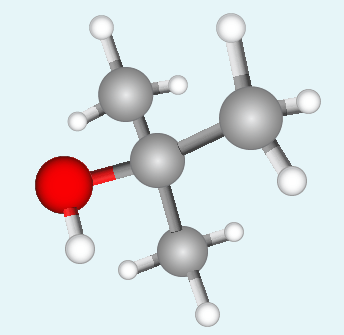
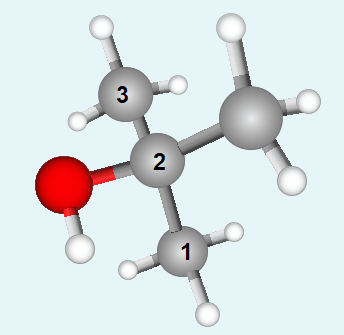
Name the tertiary alcohol below. The image shows its ball and stick model with carbon atoms represented by grey balls, hydrogen atoms by white balls and the oxygen atom by a red one.
Structure 1 |
|
Complete the table below. Table 1 Chemical formulas for some simple alcohols.
|
||||||||||||||||||||||||||||
|
What are the differences between propan-1-ol and propan-2-ol? |
Alcohols are of interest as alternatives to conventional non-renewable fossil fuels (such as coal, crude oil and natural gas). Alcohols are highly flammable and can undergo combustion. Combustion is a reaction in which a substance reacts with oxygen to give carbon dioxide (CO2), water (H2O) and heat energy. All combustion reactions are exothermic.
In particular, low molecular weight alcohols (such as methanol and ethanol) are considered attractive alternatives to fossil fuels because they can be produced at low cost (e.g., methanol can be synthesised industrially from biomass like plants, fruits and animal waste using bacteria). Additionally, alcohol-fuels are a more environment friendly energy source and their use will help to reduce emissions of greenhouse gases and toxic gases (mitigating climate change). These alcohols have the potential to become the transportation fuel instead of gasoline.
|
Write the balanced chemical equation for the complete combustion of ethanol, CH3CH2OH. |
Calorimetry
Enthalpy changes can be measured experimentally. The amount of heat energy being transferred during a chemical reaction can be measured using a calorimeter. The basic principle of calorimetry is that energy transferred during a reaction (as heat) causes the water in the surroundings to increase its temperature (for exothermic reactions) and decrease its temperature (for endothermic reactions).
In the OpenSTEM Africa Calorimetry application you will use a simple calorimeter to compare the heat energy released from burning various alcohols. The efficiency of alcohol fuels will be investigated by measuring how much alcohol is required to raise the temperature of a fixed volume of water by a number of degrees Celsius.
The calorimeter consists of a conical flask containing a known volume of water over an alcohol burner (Figure 4). The change in the temperature of water during combustion is measured using a thermometer and it is a direct measure of the alcohol energy content. The alcohol burner is weighed before and after the combustion to calculate the mass of alcohol burned in order to heat the water.

Figure 4. Simple calorimeter used to determine heat energy released in the combustion of alcohols.
|
Think about the major source of error when using the simple calorimeter shown in Figure 3. |
Calculating enthalpy changes
To calculate the energy transferred from the combustion of alcohols to the water (q) you will use the equation:
q = m c ΔT
where m is the mass of water in the conical flask, c is the specific heat capacity of water (4.18 J g-1 oC-1) and ΔT is the increase in the temperature of water.
Remember that the density of water is 1 kg/l so if you are using a volume of 100 ml of water in your experiment, this volume is equal to 100 g of water.
The experimental enthalpy of combustion of an alcohol (ΔH) will be calculated dividing the energy transferred to the water (q) by the moles of alcohol burned in the reaction (n):
ΔH = - q/ n
|
Why does the energy change (ΔH) for combustion have a negative value? |
Now work through an example calculation to check your understanding of the steps require to determine experimental values of enthalpy of combustion.
|
A spirit burner with ethanol (C2H5OH) was used to heat 100.0 ml of water. When the temperature of water had increased 40.0oC, the mass of the burner and ethanol has decreased by 0.980 g. Calculate the experimental enthalpy change of combustion for ethanol. |
Previous: Lesson objectives Next: Practical activity