3.3 Anti-viral actions of antibodies
Receptor blocking
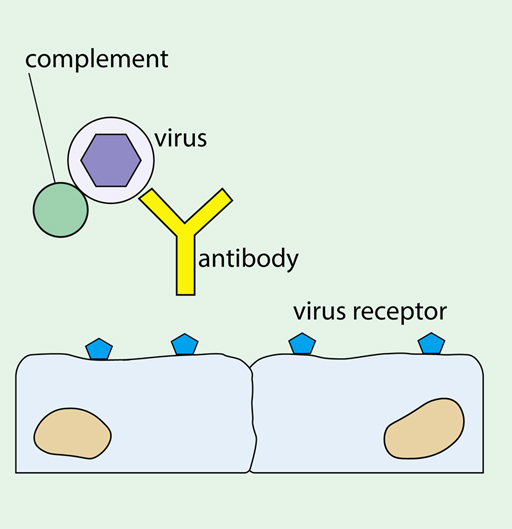
The simplest way that an antibody can interfere with virus replication is by blocking attachment to the host cell, as shown in Figure 12. Antibodies that do this are called ‘neutralising antibodies’.
-
What type of antibody would you expect to be most effective at blocking entry of SARS-CoV2 into a lung epithelial cell? Why?
-
An IgA antibody that binds to the receptor binding domain (RBD) of the spike protein. An IgA antibody will be secreted onto the surfaces of the airways in the lung. An antibody that binds the RBD will be most effective at preventing attachment of the virus to the ACE2 receptor.
Complement activation
IgM and most IgG antibodies can activate the complement system. This is a group of proteins present in blood and tissue fluids that have many functions in controlling inflammation and damaging pathogens. Antibody bound to the virus surface or virus envelope activates the complement system causing deposition of complement molecules on the surface, which you saw earlier in Figure 12 above. This can have a number of anti-viral effects, including:
- It promotes uptake and breakdown of the virus by phagocytic cells, (eg macrophages).
- It directly damages the viral envelope.
If an infected cell has virus molecules inserted in its plasma membrane, then antibodies can bind to these antigens and activate complement. Some complement components can punch holes in the target cell, thus killing it before replicating viruses can be assembled inside.
In addition, complement system molecules can attract leukocytes to sites of infection, and enhance antigen presentation and antibody production.
Promoting phagocytosis and NK cell activity
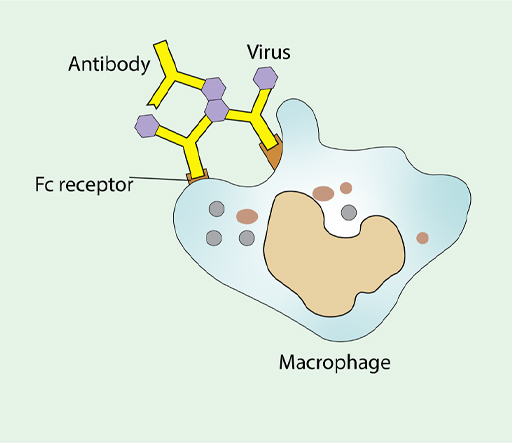
Phagocytes, such as macrophages are important in destroying pathogens. They internalise them by a different pathway than a virus would normally take. Once inside the phagocyte, pathogens are broken down by a barrage of small toxic molecules and enzymes. Antibodies play an important role in this process by acting as adapters between the virus and the macrophage, as shown in Figure 13.
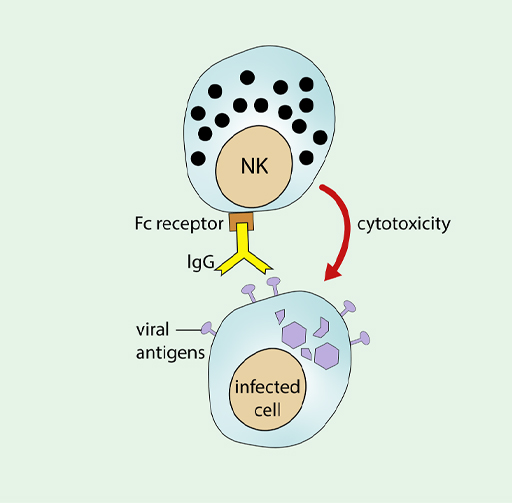
Antibody can also promote the activity of NK cells, allowing them to recognise viral antigens that have been inserted into the membrane of an infected cell, which you can see in Figure 14.
Activating intracellular defences
Until recently, it was thought that antibodies could only recognise antigens in extracellular spaces, on the cell surface or in body fluids. It has now become apparent that antibodies can also combat many viruses inside cells. Moreover, this action can be effected by antibodies that are not conventional neutralising antibodies – for example, they may bind to internal proteins of the virus.
Antibodies can enter the cell while bound to a virus, or may be taken up independently by pinocytosis, and they act in a variety of ways. Within an endosome antibodies can inhibit viral uncoating and the fusion mechanism which allows the viral genome to enter the cytosol of the infected cell. Within the cytosol, they can interfere with virus replication or assembly.
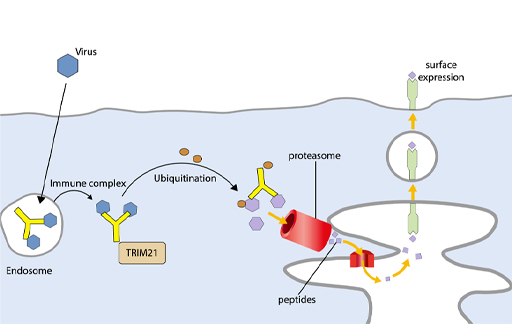
In addition, some antiviral functions are mediated by a cytosolic receptor for antibody, TRIM21 (Tripartite Motif 21) which binds the Fc portion of IgG. TRIM21 binds to a virus that has any attached IgG antibody and tags the complex of antibody and viral protein to direct it to the proteasome, where the viral proteins are degraded and can then be presented on MHC class I molecules. Thus, TRIM21 enhances antigen presentation, as shown in Figure 15. This mechanism is most relevant for viruses that enter the cytoplasm intact – i.e., non-enveloped viruses.
It is however possible for TRIM21 to target enveloped viruses that have entered the cytosol from the endosome and lost their envelope and external proteins. If antibodies against the internal viral proteins have independently entered the infected cell, then they can bind to the uncoated virus and engage TRIM21. The virus associated with an antibody in the cytoplasm as an immune complex is recognised by TRIM21 which ubiquitinates the complex. The addition of ubiquitin tags the components of the immune complex for rapid breakdown by the proteasome, so that viral peptides can be presented by MHC class I molecules.