4.1.4 Reconstituting BCG and measles vaccines with diluent
It is very important to reconstitute a powder vaccine using only the diluent provided by the manufacturer specifically for that vaccine
Reconstitution is the process of mixing vaccines that come as powders with the diluent provided with the vaccine. This section will also explain how to open vials and ampoules. BCG and measles vaccines are the two routine EPI vaccines that require reconstitution, following the steps below:
1 Wash your hands and organise your equipment and work area
Wash your hands with clean water and soap and follow the standard procedures outlined in Section 4.1. Your aim is to minimise the risk of infection or injury to yourself, your clients and their caregivers.
2 Inspect the vaccine vial or ampoule
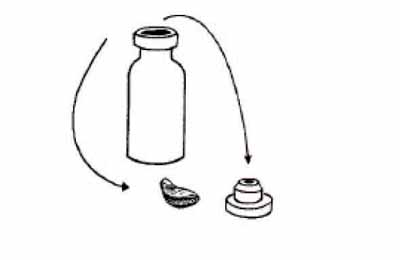
Measles vaccine powder (and most fully liquid vaccines) come in vials, but BCG vaccine powder comes in ampoules. Check that the vial or ampoule is not cracked. Check the vaccine vial monitor (VVM) and the expiry date as described above, and discard any vaccines that are no longer safe to use.
3 Tap the vial or ampoule
To make sure that all of the vaccine powder is at the bottom of the vial or ampoule, tap it with your finger.
4 Open the vaccine vial
The centre of the metal cap on a vaccine vial is pre-cut so that it can be removed easily. Lift the centre of the metal cap and bend it back, using a metal file. Some vials have plastic stoppers instead of metal caps. Flip off the stopper with your thumb. When the cap or stopper is removed, it reveals the rubber membrane on top of the vial, protecting the vaccine (Figure 4.6). How to open an ampoule of BCG vaccine is described in step 6.
5 Inspect the diluent
Most diluents for reconstituting vaccines come in sealed ampoules, which you open by breaking off their pointed tops. Check that the ampoule is not cracked and that it has been chilled to between 2ºC and 8ºC before use.
Make sure that you are using the diluent the manufacturer sent with the vaccine, and that the expiry date has not passed. Each vaccine has its own diluent and must not be reconstituted with anything else. Deaths have resulted when vaccines were incorrectly mixed with liquids other than the specific diluent approved by the manufacturer for use with the vaccine.
Even if the main ingredient of the diluent is sterile normal saline or sterile water, you must never use normal saline or water instead of the correct diluent.
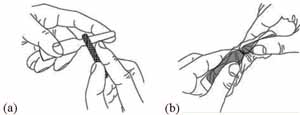
6 Open the ampoule of diluent
The process for opening an ampoule of BCG vaccine is exactly the same as for opening an ampoule of diluent.
Hold the ampoule between your thumb and middle finger, and use your index finger to support the top (Figure 4.7a). Use the metal file packed with the ampoules to scratch hard around the neck of the ampoule. Wrap the ampoule in a piece of clean cloth and gently break off the top. It breaks where you made the scratch (Figure 4.7b). If you injure your hand while doing this, discard the ampoule because the diluent may have become contaminated. Cover the wound before opening a new ampoule.
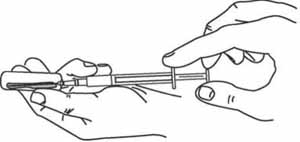
7 Draw diluent into the mixing syringe
Use a new disposable mixing syringe (0.1 ml for BCG and 1 ml for other vaccines) attached to an appropriate-sized needle. Hold the ampoule at an angle (as shown in Figure 4.8), and put the needle into the open top. Pull back the plunger to draw all the diluent from the ampoule into the syringe.
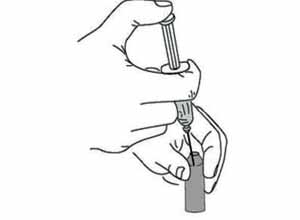
8 Reconstitute the vaccine
Insert the needle of the mixing syringe through the rubber membrane on top of the vaccine vial, or (for BCG) into the open neck of the vaccine ampoule (Figure 4.9). Push the syringe plunger in with your thumb to empty the diluent into the vaccine vial or ampoule, where it begins to mix with the vaccine powder. Draw them both up slowly into the syringe and inject them back slowly into the vial or ampoule. Repeat this mixing step several times until the vaccine powder is thoroughly mixed with the diluent.
Discard the mixing syringe and needle in a safety box and use new ones for the immunization. Ideally, you should use an auto-disable (AD) syringe for the immunization.
9 Keep reconstituted vaccines cold
Place the vial or ampoule of reconstituted vaccine into the spaces in the foam pad (a piece of soft foam that fits on top of the ice-packs; Figure 4.10) to keep it cold during your immunization session. You will learn more about the use of foam pads and ice-packs in Study Session 6.

10 Do not keep unused reconstituted vaccine
Unused reconstituted vaccine must be administered within the time limit stated by the manufacturer (usually six hours). Any unused vaccine should be thrown away after six hours, or at the end of the immunization session if you finish before the time limit has passed.
Why should you throw away reconstituted vaccines after an immunization session is completed?
Reconstituted vaccines lose their potency (strength) quickly. As you learned in Study Sessions 2 and 3, reconstituted BCG vaccine is quickly damaged by sunlight and heat, and reconstituted measles vaccine is also damaged by heat.
4.1.3 Inspecting vials and ampoules of vaccines and diluents
