6.3.3 The shake test
The shake test is how you check whether freeze-sensitive vaccines (pentavalent, PCV10, TT or HepB) have been subjected to freezing temperatures, which are likely to have damaged them. To perform the shake test, follow the steps below:
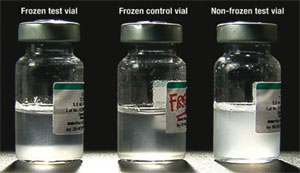
Step 1 — Prepare a frozen control vial: Take a vial of vaccine of the same type and batch number as the vaccine you want to test, and from the same manufacturer. Freeze the vial until the contents are solid (at least 10 hours at –10°C) and then let it thaw. This is the frozen control vial (the middle vial in Figure 6.10). Mark the vial clearly so that it is easily identifiable and will not be used by mistake.
Step 2 — Choose a test vial: Take a vial (or vials) of vaccine from the batch that you suspect has been frozen. This is the suspected frozen test vial (on the left in Figure 6.10).
Step 3 — Shake the control and test vials: Hold the frozen control vial and the suspected frozen test vial together in one hand and shake them vigorously for 10–15 seconds.
Step 4 — Allow the vials to rest: Leave both vials to rest by placing them on a table side by side and not moving them further. A freeze-sensitive vaccine that has not been frozen appears as a uniformly cloudy liquid (see the vial on the right in Figure 6.10). After freezing, the vaccine tends to form flakes that quickly settle at the bottom of the vial to form a sediment when you leave it to rest after vigorous shaking. The speed at which the flakes settle is called the sedimentation rate. Note that some vials have large labels that conceal the vial contents. This makes it difficult to see the sedimentation process. In such cases, turn the control and test vials upside down and observe sedimentation taking place in the neck of the vials.
Step 5 — Compare the vials: Observe the difference in sedimentation rates in the frozen control and suspected frozen test vials for a maximum of 30 minutes. View both vials against the light to compare the sedimentation rate. If the vaccine in the suspected test vial shows a much slower sedimentation rate than the vaccine in the frozen control vial, you can conclude that the test vaccine has most probably not been frozen and can be used.
What should you conclude if the sedimentation rate is similar in the suspected test vial and the frozen control vial?
You should conclude that the test vial has probably been damaged by freezing and the vaccine should not be used.
The shake test should be conducted for all vaccines with the following characteristics:
- Vaccines packed in boxes that have a freeze indicator (e.g. freeze tag, see Figure 6.9), which is found to be activated.
- Refrigerator temperature records that show the temperature has fallen below +2ºC.
- Where you suspect that the vaccines may have been frozen by mistake, for example by placing too close to the freezer plate in the refrigerator, or touching frozen ice-packs.
If the vaccine fails the ‘shake test’ it must be discarded. There is no need to conduct a shake test if a liquid vaccine vial is already frozen solid — simply discard it. Also discard any vials that develop white lumps of sediment attached to the glass, which cannot be dispersed despite vigorous shaking. This can happen if pentavalent vaccine is exposed to freezing below 0ºC.
6.3.2 Freeze indicators
