Principle of anodising
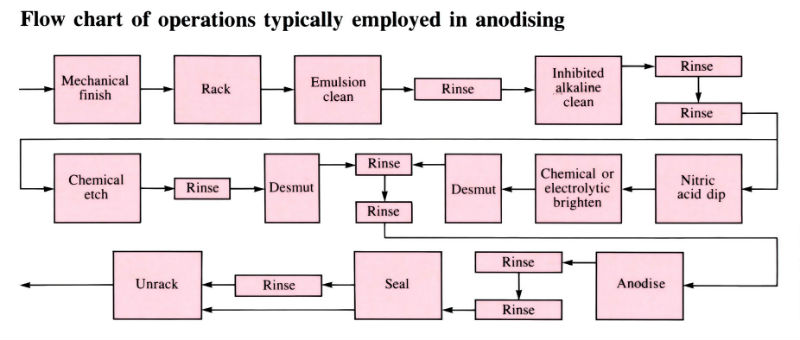
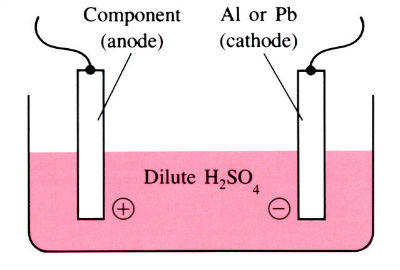
Aluminium and its alloys have some inherent resistance to atmospheric corrosion due to the presence of a very thin protective oxide film 2.5–10 nm thick. Anodising is an electrolytic process for producing much thicker oxide coatings, 5–30 µm thick, with improved physical and chemical properties. The components are made the anode in a dilute acid solution. The oxygen liberated at the anode face results in the formation of a coherent oxide film which is very adherent to the base metal. Process involves several pre-treatment steps, as shown in the flow chart below.
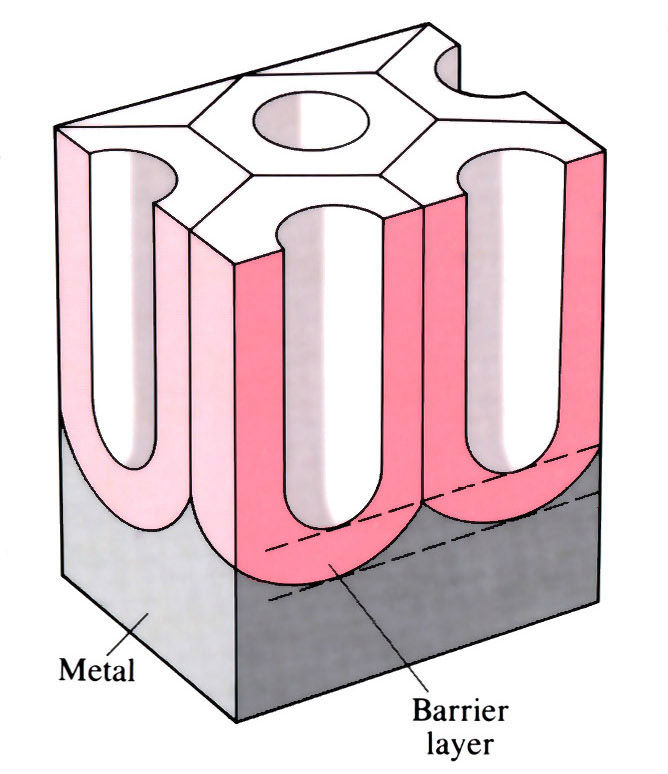
Microsection of an anode film showing porous cell structure
If the electrolyte has some solvent action, then a porous film is produced and oxidation can continue, giving thick films. The film consists of hexagonal columns, each with a central pore which reaches down to a thin compact barrier layer.
Manufacture:
- Before any pre-treatment is given to the aluminium component, it is very important to consider its initial surface condition in relationship to its final application. Components should be free from swarf and grit and should not be damaged due to handling.
- Any machining, drilling and welding should be carried out before anodising.
- The final appearance of the component is largely dependent on the type of pre-treatment carried out before anodising.
- Types of pre-treatment range from mechanical processing such as “abrasive polishing” to chemical treatments such as “chemical brightening” or “electrolytic polishing”.
- The type of plant ranges from small manual load and unload, up to fully automatic in-line plant ranging in amperage capacities from 500–80,000 A. Computer controlled units are used for long lengths of aluminium extrusion.
- Titanium is used for jigging.
- Electrolyte solutions are of three main types:
- 10–15% sulphuric acid process at 20–25°C; rate of film formation 25 µm h-1
- sulphuric acid-oxalic acid at 30°C; rate of film formation 30 µm h-1
- 10% chromic acid at 38–42°C; rate of film formation 15 µm h-1
- Hard anodising is used to give thicker (25–100 µm) and harder (500–900 VPN) wear resistant surfaces to aluminium and its alloys – uses H2SO4 – oxalic acid, at higher volumes at 0–10°C (refrigeration).
Materials:
- The majority of anodising is done on aluminium and its alloys.
- The type of finish is governed by the final application and alloy composition.
- If mechanical properties are the most important feature, then the choice will be limited. Where resistance to atmospheric corrosion is the prime requisite, then it is often possible to select alloys giving coatings that combine good protection and an attractive appearance. Most applications are for decorative purposes, and here the choice of alloy is the most extensive.
- 99.7% pure aluminium gives good bright anodising properties for small reflectors.
- The Al-(0.6-0.8%) Mg-(0.3-0.6%) Si alloys give good anodised finishes on extrusions and forgings.
- The quality of anodised finish on castings depends on the casting method used, i.e. sand, gravity or die casting. Only a small range of alloy compositions can be used for decorative purposes, because the Al-(6-12%) Si alloys, mainly used for die casting, become grey after anodising, due to the high silicon content.
- Other materials which can be anodised include magnesium and titanium alloys.
Design:
- Designing for anodising depends on knowledge of the effects of the method of manufacture, and surface pre-treatment before final anodising.
- Castings can reveal pronounced and sometimes unsightly grain structures, while forgings reveal the flow pattern of the deformed metal.
- Sand blasting produces an even matt surface before anodising.
- Mop brushing produces a lined texture, and satin finishes can be obtained with revolving stainless steel wire wheels.
- The filler rod used for welding must be selected so that its appearance after anodising closely matches the parent metal. It is difficult to disguise the cast weld pool.
- In order to avoid trapped electrolyte, design should avoid:
- Blind holes (especially less than 6 mm dia.)
- Closed beaded edges on spinnings.
- Very narrow (less than 2 mm width) channels in extruded sections.
- Holes are drilled in long extrusion channels for drainage of electrolyte.
- Jigging points should be arranged on non-significant surfaces.
- A wide range of colour-dyed coatings can be achieved ranging from yellow to dark blue, red and black.
See also: Electroplating, Electroless plating, Phosphating, Ion implantation and Chemical vapour deposition (CVD).
This article is a part of Manupedia, a collection of information about some of the processes used to convert materials into useful objects.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews