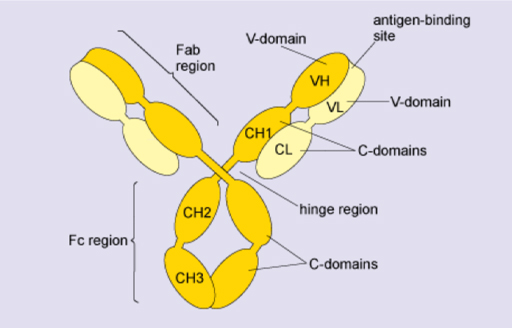
3.1 Antibody classes
Secreted IgG is produced as a monomer of the basic antibody structure (2 heavy chains, 2 light chains) but IgM is a pentamer and IgA is a dimer, as illustrated in Figure 9 and 10 below.
In Figure 9, the four polypeptide chains of an IgG molecule are folded into domains. The variable (V) domains of the heavy and light chains (VH, VL) form the antigen-binding sites. The remaining domains are relatively constant (C domains). The hinge confers segmental flexibility.
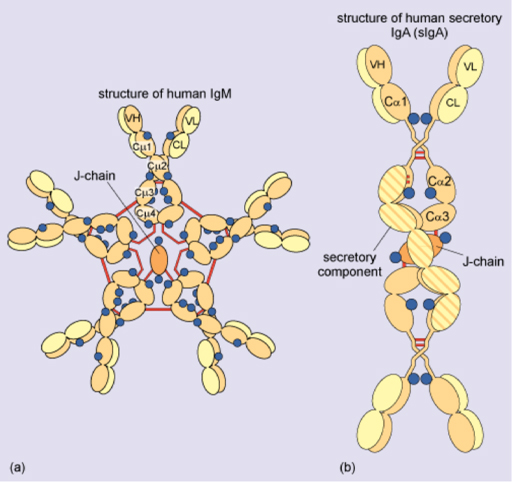
In Figure 10, each molecule is joined by an extra joining (J) chain. When IgA is secreted, it has an additional chain, the secretory component. (Note: carbohydrate units are shown in blue and inter-chain disulphide bonds in red.)
-
How many antigen-binding sites does IgM have?
-
Ten
The multiple antigen-binding sites of IgM make it very efficient at cross-linking antigens and therefore useful as the first antibody produced in an immune response. However, the affinity of IgM for its target antigens is lower than the affinity of IgG and IgA antibodies produced later in an immune response.
You will now look at how antibodies can protect against virus infection.