1.5 Experiment 2: Cucumbers and osmosis
In the previous experiment, you determined the water content of a potato and illustrated the rate at which the water is driven off in your graph. You also developed skills in carrying out an experiment. You’re now going to carry out a second experiment looking at the way water gets in and out of cells.
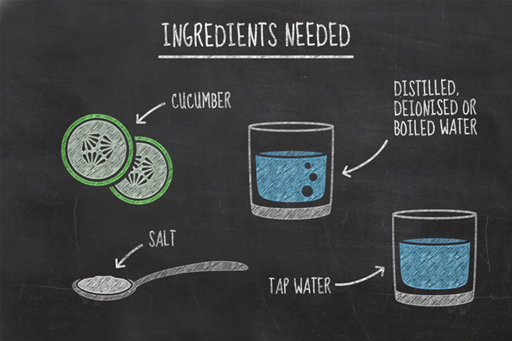
In this experiment, you will be measuring changes in the water content of two slices of cucumber as they are left in two different liquids; distilled water and salty water.
To carry out this experiment, you will need:
- two slices of cucumber
- two glasses
- a knife
- a peeler
- tap water
- distilled, deionised or boiled water
- two tablespoons of salt.
It is best to use distilled water for this experiment, available from most petrol stations and car spares shops. Distilled water is simply water that has had most of its impurities removed by boiling it, then collecting the steam and condensing it in a clean container. An alternative is deionised water, sometimes called demineralised water. This is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead.
While it’s okay to drink small quantities of distilled and de-ionised water, we don’t recommend it. Why do you think the purest form of water might not be good for you? Perhaps you’ll be able to see why at the end of the experiment.
Follow Janet’s instructions in the video (or use your activity booklet [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] PDF) and remember to keep clear and accurate notes in your journal. Once again, think about the variables that could affect your results.

Transcript
Based on her initial findings, Janet decided to change the parameters of her experiment and leave her cucumber slices overnight. You may find that you have to do the same. If so put the experiment somewhere where no-one can knock it over, and no pets try and drink it.
You’ll have the opportunity to discuss your findings in the next section.
