1.2 Units of mass
You will have noticed that the term mass in the title of this section. Mass is a measure of how much matter an object contains, and is the correct term to use rather than weight. However the terms weigh and weight are used in general day to day parlance.
Weight [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] is actually the gravitational force experienced by an object (measured in newtons):
Weight = mass × acceleration due to gravity
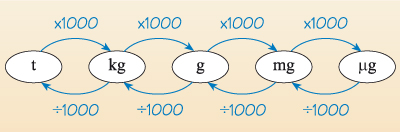
Mass is measured in kilograms (kg), which again is not the most appropriate unit for laboratory use; chemists tend to weigh in grams (g, one thousandth of a kilogram) or milligrams (mg, one thousandth of a gram).
To help you visualise these masses, consider the following:
- A litre of water weighs 1 kg.
- A £20 note weighs about 1 g.
- A UK pound coin weighs around 10 g.
Originally a ‘pound’ was the monetary value given to a pound mass of sterling silver (an alloy of 92.5% silver and 7.5% copper), hence ‘pound sterling’.
Express 120 mg in grams
120 mg = 0.120 g
In practice, the most commonly used units differ from each other by a factor of 1000. For example, in Figure 1 you can see how the units of mass – tonne (t), kilogram (kg), gram (g), milligram (mg) and microgram (µg) – differ from each other by a factor of 1000.