2.4 Inhibitors of metabolic reactions
Antibiotics that disrupt essential bacterial metabolic pathways are acting as
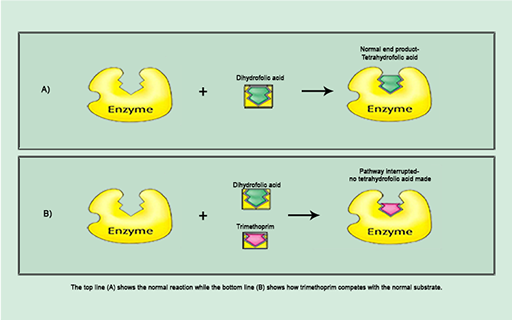
For example, trimethoprim inhibits the synthesis of folic acid, a vitamin which bacteria, unlike humans, must make themselves. Trimethoprim is a
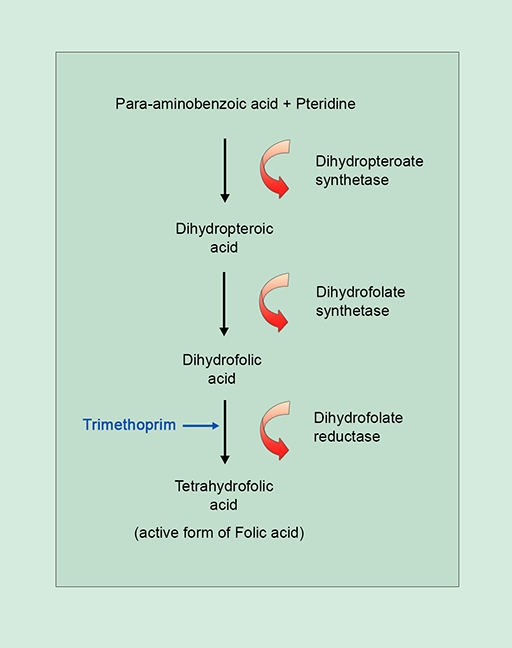
The action of trimethoprim illustrated in Figure 7b exemplifies the specific interaction between antibiotic and bacterial target at a molecular level which disrupts a particular cellular process. You will return to this topic in Week 3 in relation to the development of antibiotic resistance.
In the next section, you will look in detail at the mechanism of ß-lactam antibiotics.