3 Case study: mechanism of ß-lactams
The β-lactam antibiotics target the bacterial cell wall (Figure 8) by inhibiting the enzymes responsible for cross-linking adjacent molecules in the peptidoglycan layer. The ß-lactam antibiotics bind to these enzymes, collectively known as
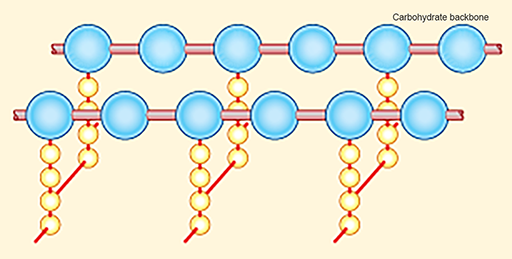
In Section 2 you learned that the activity of penicillins and cephalosporins resides in the ß-lactam ring. Activity 3 looks more closely at this.
Activity 3 Mechanism of ß-lactam antibiotics
First, watch the short video below which describes the inherent instability of the ß-lactam ring structure which makes it highly reactive. The video refers to penicillin, but the same is true of the ß-lactam ring in cephalosporins and all other antibiotics of this class.

Transcript: Video 4 The inherent instability of the ß-lactam ring structure.
Figure 9 shows what happens when a ß-lactam antibiotic, in this case penicillin, binds to an active PBP.
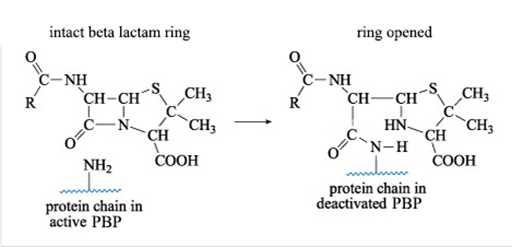
The reaction shown in Figure 9 results in a new PBP side chain which is much larger than the original -NH2 group and effectively deactivates the PBP. Can you suggest why?
Answer
The large side chain means that there is no longer sufficient space for the enzyme (PBP) to bind to its normal substrate during the peptidoglycan cross-linking process.
You will learn more about how disrupting the interaction between ß-lactam antibiotics and PBP contributes to antibiotic resistance mechanisms in Week 3. Next, however, you will see how antibiotics of the same class, and with the same mode of action, can have a different spectrum of activity and exert different effects.
