1.2 Destroying or modifying the antibiotic molecule
The second mechanism of antibiotic resistance you will look at is the destruction or modification of the antibiotic by bacterial enzymes. Probably the most well studied example of enzymes that destroy antibiotics are the β-lactamases
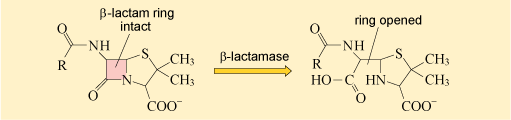
As you may recall from Week 2, β-lactamases deactivate the β-lactam ring of β-lactam antibiotics, preventing them from binding to their target (Figure 3).
The β-lactamases can deactivate almost all of the β-lactam antibiotics currently in therapeutic use. As you will see in the case study at the end of this week, this includes cephalosporins. Consequently, their presence significantly reduces the available treatment options for infections caused by bacteria expressing
How might a β-lactamase inhibitor help the treatment of infections caused by β-lactamase-expressing bacteria?
The β-lactamase inhibitor will block the ability of the β-lactamase to deactivate the β-lactam antibiotic so that it can bind to its target molecule.
Other antibiotic-modifying enzymes do not destroy or target the core chemical structure that confers antibacterial activity. Instead they modify the antibiotic’s structure by adding chemical groups to prevent it from binding to its target. One group of antibiotics that are particularly susceptible to modification are the aminoglycoside antibiotics which include streptomycin (Figure 4).
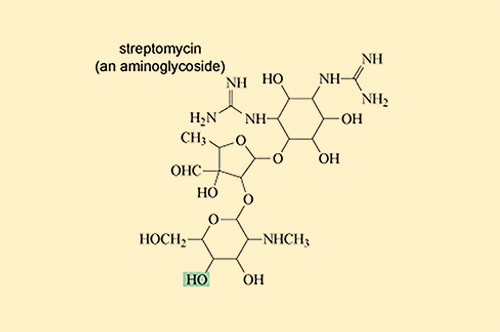
Aminoglycoside-modifying enzymes add bulky chemical groups to the exposed hydroxyl (-OH) and amino (-NH2) groups of the antibiotic, which prevent it from binding to its target.