3.3 Chemical composition
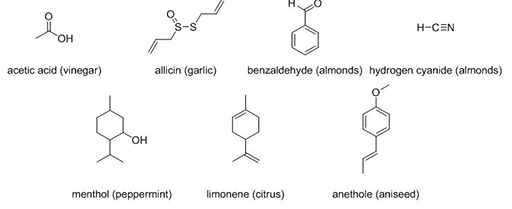
The chemical composition of a molecule is also really important. The seven compounds in Figure 5 all have familiar odours.
Compare menthol and limonene. What can you say about their structures and the odours they elicit?
Menthol and limonene have quite similar structures. The only major differences are the presence of double bonds (shown as =) in limonene and the C–OH in menthol and yet they have very different smells.
Now compare benzaldehyde (C7H6O) and hydrogen cyanide (HCN), both of which have an almond smell. What molecular features do they have in common?
Benzaldehyde has a higher RMM than hydrogen cyanide. Not only are the masses very different, so are the structures. Benzaldehyde contains a ring of carbon atoms and a C=O group whereas hydrogen cyanide is linear and contains a C≡N group. There appears to be no common feature, other than the C–H bond, that could be considered to cause an almond smell.
All of the compounds in Figure 5 exhibit medium–high volatility. They also have vastly different structures. Some contain different atoms (C, S, O, N) than the others, and some have ring structures while others are linear. It is the presence of these different atoms that controls how odorants interact with specific compounds known as smell receptors located in the nasal cavity, but also the kinds of smell that the molecule elicits. It is this interaction of the odorant with the smell receptors that elicits the smell sensation. As examples, odorants which contain amino groups (–NH2) generally have a fishy smell and odorants with ester functional groups (–OCOC) are usually fruity.
A direct consequence of the chemical composition of an odorant molecule, is the ability of the odorant to interact with other molecules around it (for example, water or other chemicals in the air) via a specific type of bonding known as hydrogen bonding. The odorant will exhibit slightly different properties as it associates with different molecules.
The key point to take away here is that, while the properties of an odorant seem limiting (small and volatile), the truth is actually the opposite. The complexity of the available chemistry that can make such molecules is vast, leading to thousands of potential odorant compounds, all of which are recognised by the body.