3 The process of distillation
As you have just discovered, the process of distillation is fundamental for the production of gin. Distillation is an example of a chemical process that is commonly utilised both in a laboratory setting and also on massive industrial scales – such as for gin production!
By definition, distillation is a process that exploits differences in the volatility of the components of a mixture. It is widely used both on a laboratory and an industrial scale to separate the components from a liquid mixture by selective boiling and condensation. Different components of a mixture have different boiling points, and it is these differences which enable the process of distillation to perform the separation.
So, distillation may be applied for the complete separation of pure components, or for partial separation to control the concentration of selected components within the mixture.
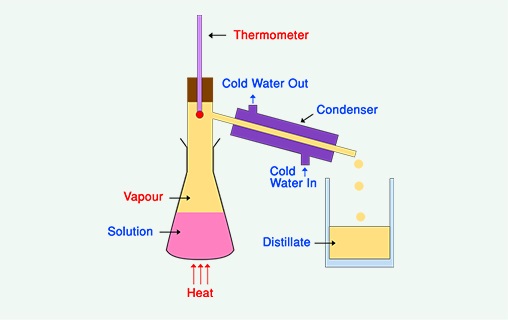
A typical apparatus set-up is illustrated in Figure 2.
Distillation is commonly used to separate ethanol – the alcohol in alcoholic drinks – from water. An example of such a distillation is illustrated in the following video on a laboratory scale.

Transcript
LOUISE MACBRAYNE
Here we have an example of a typical distillation apparatus that we would use in a laboratory. We can use this setup to demonstrate the principle of distillation that would, in fact, be used on a commercial scale to produce our spirits. So in a typical distillation setup, we have a heating source here, because we need to be able to raise the temperature of the substance that we're going to distill to its required boiling point. So we have our heating source, and into this heating source, we're going to place the substance that we're going to distill. We then have an adapter, because we need a thermometer to measure the temperature at which our distillation is occurring. Our adapter then links into this piece of apparatus here, which is known as a condenser. We use tubing to basically run cold water around the outside of the condenser. And that's going to cool the vapour that we've produced by boiling and allow it to condense into our pure liquid here that we're going to collect in this receiving flask. So in this particular setup, we have our mixture here of ethanol and water. And because ethanol and water have different boiling points-- ethanol boils at a lower temperature than water, it boils at approximately 78 degrees Celsius-- we can start to heat our mixture. When we reach the boiling point of ethanol, only the ethanol will start to boil. Our water isn't hot enough to boil. Therefore, by boiling and condensing, we can remove our ethanol, our pure ethanol, from this mixture here. So we're going to turn the temperature on now, leave it, and start watching for bubbles to form within our liquid, because that's going to tell us when our distillation process is beginning. Our laboratory still has now been running for about 20 minutes. And if we look, we can see that something's happening. We've got bubbles here in our starting reaction, and we're starting to see a clear liquid being collected here in our receiving vessel. So if we cast our eye now to our thermometer and take a reading, we can see that our thermometer is recording a temperature of about 85 degrees Celsius. So what's happening in here, in our starting material, is that our particular substance that has a boiling point which corresponds to what we're reading on the thermometer is what's boiling, and therefore, being collected in our receiving flask. And that substance is ethanol, because the temperature here is not sufficient to boil the water. So it's only our ethanol that's causing the bubbles here. Our ethanol is boiling. The vapour here is being collected into the adapter, and the temperature of the vapour is being picked up by the thermometer. It's condensing and being collected here. And you can see here, we have a clear, colourless liquid, which is our pure ethanol that has been distilled out of our starting material. So in this process, we've utilised a very common purification technique that we use in chemistry. We've used distillation to separate out the ethanol from our starting mixture, purely on the basis of its boiling point.
Considering what you have observed in the video, answer the following questions.
In a mixture of ethanol and water, which will evaporate first upon heating from room temperature?
Ethanol has a lower boiling point than water, so it evaporates first. The ethanol vapour is then cooled and condensed inside the condenser to form a pure liquid known as the distillate.
How do you know when it is ethanol that is boiling in the distillation process?
Assuming you know that the boiling point of water is 100°C, and you know that the solution contains only ethanol and water, when you start to see the distillate collecting in the receiving flask, you can read the temperature corresponding to the boiling point of this substance from the thermometer. If this temperature is lower than 100°C then the distillate must be something other than pure water, in this case it will be the ethanol.
Arrange the following stages into the order in which they occur during the distillation process: condensation, evaporation, heating, cooling
Heating – evaporation – cooling – condensation.
Distillation of alcohol can be done anywhere, whether it's in the home or a laboratory, but in most countries it is illegal to distil alcohol without a licence. You may have heard illegally-distilled alcoholic drinks referred to as ‘moonshine’. You will learn more about counterfeit alcohol in Week 8.