1.3.1 Enzymes – biological catalysts
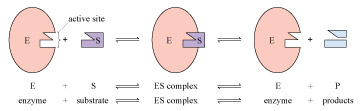
An enzyme is a protein molecule that has the ability to accelerate a particular chemical reaction in a cell, allowing it to take place at body temperature. An enzyme remains unchanged at the end of the reaction: in essence, a biological catalyst. In a cell, enzymes bind to their target molecules (substrate) and act to facilitate the chemical reaction for which they are responsible. During this process, enzyme–substrate complexes are formed. The enzyme-substrate complex then releases the product(s) of the reaction, in doing so reforming the active enzyme which can the move on to the next substrate molecules. The overall reaction between an enzyme and its substrate is illustrated in Figure 2.
Thinking back to Week 2 and the brewing process, in what steps are enzymes involved?
Enzymes are important in the processes of malting and mashing, to convert the starch present in the barley into simple sugars which can be utilised by the yeast during the fermentation process. The yeast itself also has enzymes which are involved in the fermentation process when the sugars are used to produce alcohol.
In the cells of the liver, ethanol is first converted to acetaldehyde through a chemical reaction facilitated by a liver enzyme called alcohol dehydrogenase (ADH).
Acetaldehyde is a more toxic chemical than ethanol and, if it builds up in the bloodstream because of excess drinking, it causes people to feel very unwell, with symptoms such as vomiting, headache, rapid heartbeat and flushing (redness) of the skin of the face, neck and shoulders. However, acetaldehyde is not normally present in the body for very long because it is quickly converted by another liver enzyme called aldehyde dehydrogenase (ALDH) into acetic acid (a molecule which you may know better as the main component of vinegar), which is a non-toxic molecule in humans. Acetic acid is converted to a useful molecule called acetyl-CoA which then enters one of the body’s regular biochemical cycles, for example in the process of aerobic respiration, to produce energy.
Box 2 Why is it some people can handle their drink better than others?
Individuals can have differing amounts of these enzymes in their body, but they can also have different versions of them as well. These different versions are called isoforms. Essentially, they are enzymes that are very similar to each other, but there are small differences in their structure that may affect the way in which they catalyse the reaction they are responsible for. The production of all proteins in the body – including enzymes – is controlled by genes, individual units of the inherited genetic material DNA. Everyone inherits from their parents a complete set of genes, including those that direct the production of the ADH and ALDH enzymes.
ADH exists in five different isoforms in the human population. Two of the five types of ADH metabolise ethanol to acetaldehyde more rapidly than the other three, resulting in the accumulation of higher amounts of acetaldehyde and making a drinker who possesses either of these forms feel uncomfortable more quickly – even a small amount of ethanol can make them feel very ill. These particular ‘fast acting’ isoforms of ADH are common in people of Asian origin, who therefore tend to accumulate high levels of acetaldehyde when they drink. More than 75% of Japanese people who drink report flushing (a redness of the face) compared to 5–10% of Caucasians. On the other hand, a positive effect of these unpleasant symptoms is that they help to protect people of Asian origin from developing alcohol use disorders.
In some people, irrespective of the isoform present, an insufficient amount of ADH is produced in the liver, and so such people will only be able to metabolize ethanol very slowly, causing the ethanol to remain in the system longer, thus prolonging intoxication. The level of ADH is also reduced as people get older.
The overall rate of ethanol metabolism depends on many factors, and the rate at which the blood–alcohol concentration (BAC) falls after drinking can range from less than 10 mg per 100 ml per hour to over 40 mg per 100 ml per hour for different people.