2.2 Principles of IR spectroscopy
You have just learned about the detection of alcohol by a chemical technique. However, an alternative method can be used for this purpose both for in situ roadside testing and also in the laboratory.
Earlier, you looked at mass spectrometry. Another very commonly-used analytical chemistry technique is infra-red spectroscopy, which is based on the absorption by a molecule of a particular type of light.
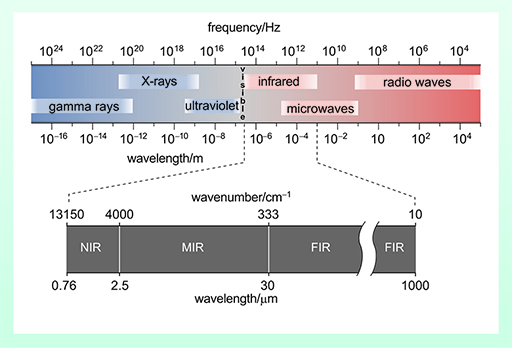
In the 1670s, Sir Isaac Newton (1643–1727) determined that light is composed of a spectrum (rainbow) of colours. Nowadays we refer to this spectrum of colours as the electromagnetic spectrum, as illustrated in Figure 7.
In 1800, a German astronomer called Sir Frederick William Herschel (1738–1822), most famous for the discovery of the planet Uranus, discovered the part of the electromagnetic spectrum known as the infra-red region when he was observing the Sun. In building his telescopes, Herschel began experimenting with different coloured filters which could help him observe the Sun. While using a red filter to reduce the Sun’s glare, he noticed that heat was generated, and these rays he deemed to be ‘calorific rays’ – later these were renamed infra-red (IR).
IR spectroscopy detects the absorption of light by a compound, in the IR region of the electromagnetic spectrum. To absorb light a molecule must have a bond within its structure that can exhibit what is referred to as a ‘dipole moment’ which means electrons within a bond are not shared equally.
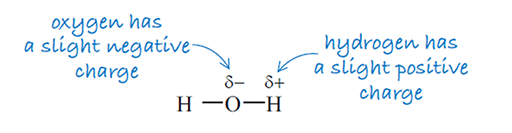
Revisit the structure of water from Week 1 in Figure 8 below.
Electrons within the O–H bond are not equally shared meaning that the electrons are more closely attracted to the oxygen than the hydrogen. As electrons are negatively charged, this leads to the O of the bond bearing a slight negative charge and the H a slight positive charge. It is this charge difference which enables the IR light to be absorbed by this particular bond, giving rise to a peak within an IR spectrum.
Different bonds within functional groups which possess a dipole moment will absorb at different areas of the IR spectrum, referred to as wavenumbers.
An IR spectrometer is a relatively simple device consisting of a lamp or heated rod that will emit light in the IR region. A detector then collects all wavelengths of IR radiation that have passed through the sample and converts these to wavenumbers. Each chemically distinct molecule will have a different absorption pattern made up from the number and different types of bond present and the presence of differing functional groups.