1.1 What is alcohol?
To start with it is important for you to know exactly what alcohol is. This will make it easier as you read through the course and will facilitate a clearer understanding of the science, as the term ‘alcohol’ has both a generic and a specific meaning.
ITQ _unit2.1.1
-
What do you understand by the term ‘alcohol’?
-
You may have considered any of the following images as defining, or being associated with, alcohol (Figure 1).
In general the word alcohol is associated with an alcoholic drink, but we need to be more specific than this. In scientific terms an alcohol is a compound (a group of atoms that make a molecule) that contains ahydroxyl group (–OH) bound to a carbon atom (C). Figure 2 has some examples of these alcohols to show you some of the structures of these chemicals.
When describing a chemical structure, the terms ‘bond’, ‘bound’ and ‘binding’ are used to discuss how atoms and molecules interact with each other. You don’t need to know the details at the moment; just be aware of the terminology.
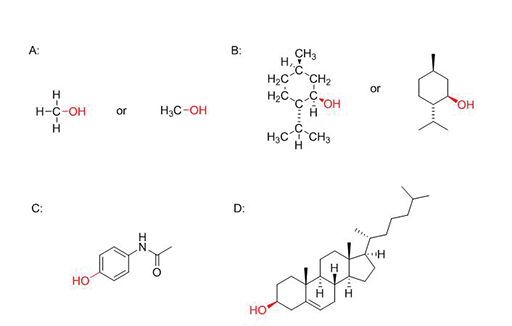
Figure 2 depicts the following alcohol compounds:
A: Methanol. This is the simplest alcohol containing a single carbon atom, three hydrogen atoms and the hydroxyl group (an oxygen and a hydrogen atom). On the left the full structure is shown. The carbon atom and the three hydrogens together form a ‘methyl’ group which can otherwise be written as CH3 or H3C so that the group is joined to the rest of the compound via the C atom.
B: Menthol. This natural compound is found in mints. This compound interacts with the human body as a mild anaesthetic and has a cooling sensation. It has a number of different atoms bound together. The left-hand figure shows the full structure but it is easier for chemists to simplify it as shown on the right, where the 6-sided ring structure is a much simpler way to represent the arrangement of carbon and hydrogen atoms.
C: Paracetamol. This common drug is used to treat pain and fever. It is classed as one of the world’s essential medicines but, like many drugs, it is dangerous in large amounts. Note that N denotes an atom of nitrogen.
D: Cholesterol. This is a natural compound found in animal cells and is an essential molecule for life, forming the basis for some hormones and other important chemicals. High levels of cholesterol in the blood can lead to cardiovascular disease.
So all the compounds shown in Figure 2 are technically alcohols.
ITQ _unit2.1.2
-
What do the names of these four compounds have in common?
-
They all end in –ol. In fact, if the name of any compound ends with –ol then it will have a hydroxyl (–OH) group in it and be an alcohol.
The ‘alcohol’ that is referred to in drinks is one of this family of similar chemicals containing an –OH group, and the particular one that is present in alcoholic drinks has the chemical name ethanol.

