1.4.1 Principles of mass spectrometry
Mass spectrometry is an important analytical method and a major tool for analysing and identifying unknown compounds.
If you subject a moving object to a sideways force, the object will be deflected away from its original straight-line path, into a curved path. You can apply exactly the same principle to atomic-sized particles in order to measure their mass.
Atoms and molecules can be deflected by magnetic fields – provided the atom or molecule is first turned into an ion. Electrically-charged particles are affected by a magnetic field but electrically-neutral ones aren't. You were first introduced to ions in Week 1 of this course.
ITQ _unit9.1.2
-
What is the charge on an atom if a negatively charged electron is removed?
-
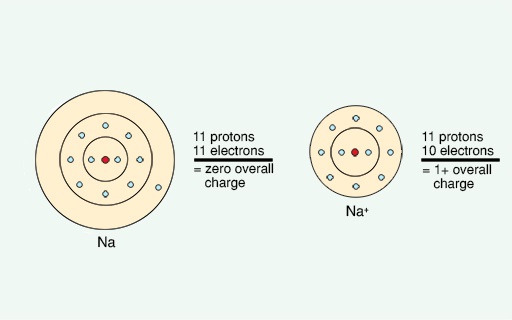
If an electron is removed, the atom no longer has an equal number of protons and electrons. There will be an excess of one positively-charged proton in the nucleus of the atom. The atom will now be a positively-charged ion because the proton is not being neutralised by the presence of an electron. This is illustrated in Figure 4 for the metal sodium.
Sodium is a highly reactive metal and one you may unknowingly be very familiar with already.
ITQ _unit9.1.3
-
What common household substances contain sodium?
-
Sodium is a key constituent of common table salt (as sodium chloride, NaCl), baking soda (as sodium bicarbonate, NaHCO3) and of bleach (as sodium hydroxide, NaOH).
Sodium has an atomic number of 11. This means that it has a total of 11 positively-charged protons in the nucleus. The positive charge associated with these protons is balanced by 11 negatively-charged electrons in shells which orbit around the nucleus (a bit like the rings of the planet Saturn). A very reactive metal such as sodium will readily lose an electron - you now have 11 positively-charged protons in the nucleus but only 10 negatively-charged electrons orbiting it. The charge is no longer balanced, so sodium is now positively-charged and has become an ion. Less reactive metals such as copper or silver will not readily lose electrons and hence are used in every day objects such as jewellery or coins.
Molecules of different masses and structures will produce different ions, and hence different mass spectra, which can be used to identify them.
So, methanol and ethanol will each produce different mass spectra because these two alcohols are different compounds with different masses. If you separate a sample of alcohol containing both ethanol and methanol into its constituent components, and subject each component to mass spectrometry, you should be able to differentiate between a methanol contaminant and the ethanol that you would expect to see.
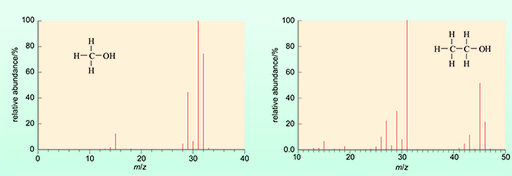
The characteristic mass spectrum of both methanol and ethanol are illustrated in Figure 5 below.
ITQ _unit9.1.4
-
What do you notice about the complexity of the two spectra?
-
The ionisation of methanol produces a spectrum with far fewer peaks.
Note that the detector of the mass spectrometer has counted the ions as they are generated to give a percentage relative abundance of ions with particular m/z (mass/charge) values.
In addition to detecting methanol in alcoholic beverages, mass spectrometry can also be used to detect and quantify other contaminants, for example undesirable substances such as pesticides. Heavy metals (for instance lead, mercury and arsenic) are also detectable.