2.4 Bauxite – amazing things that a lot of rain can do
Sometimes weather is all it takes to form an ore, so long as the weather is intense enough and you give it enough time!
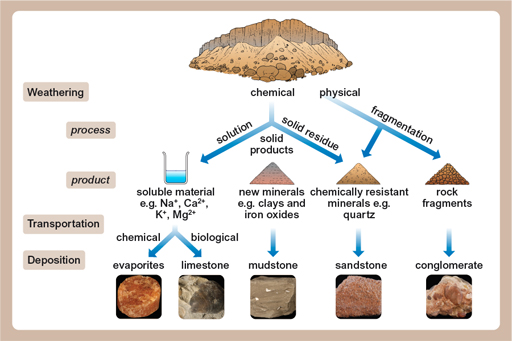
Bauxite is an aluminium ore that forms just from the weathering of rocks. Weathering is what happens when rocks are left out in the rain, and comes in three forms: physical, chemical and biological. Physical weathering is when bits of rock are attacked by wind, or water, or sometimes just gravity during rock falls. Chemical weathering is when rocks slowly dissolve – rainwater is naturally ever so slightly acidic, and that acid will slowly dissolve the rocks. The third sort of weathering is biological, where rocks are attacked (chemically or physically) by living things.
For bauxite to form, the important factor is chemical weathering under very specific conditions. Aluminium is the third most abundant element in the Earth’s crust (after oxygen and silicon) and, at 8.4%, the most abundant metal. Aluminium is usually combined very tightly with other elements in some very common minerals, so although there’s a lot of it almost everywhere, it’s very expensive to extract.
Fortunately, the chemical weathering of one of those minerals, feldspar (found in lots of igneous and metamorphic rocks), forms kaolinite, a clay mineral. This process increases the amount of aluminium oxide from less than 28% (in feldspar) to 40% – but that’s still not enough for it to be worthwhile to extract it. However, in really warm climates (like the tropics), the kaolinite is chemically weathered even more, forming minerals like gibbsite, which has high enough concentrations to be worthwhile to mine and process.
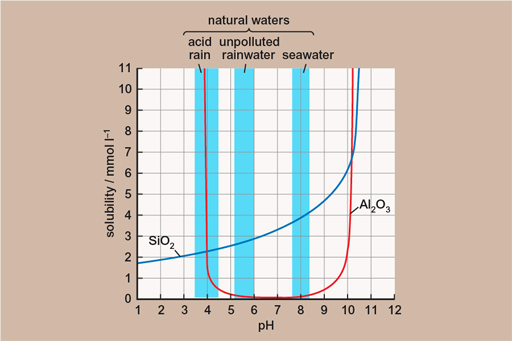
The essential thing in the formation of bauxite is that all of the other things which aren’t wanted (like silica – silicon dioxide, quartz, is its best known form) can, in tropical conditions, stay dissolved in the rainwater and be carried away, leaving the aluminium in place. This effect is shown in the figure below – showing the solubility of the aluminium oxide (Al2O3) and silica (SiO2) at different pH levels (a measure of acidity). This means that given enough time, enough starting material and enough weathering, you can go from having a lot of rock with not very much aluminium in it to having not much rock with a lot of aluminium in it!